Newer human inosine 5'-monophosphate dehydrogenase 2 (hIMPDH2) inhibitors as potential anticancer agents
- PMID: 29792360
- PMCID: PMC6009919
- DOI: 10.1080/14756366.2018.1474211
Newer human inosine 5'-monophosphate dehydrogenase 2 (hIMPDH2) inhibitors as potential anticancer agents
Abstract
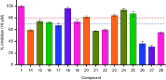
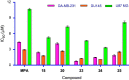
Human inosine 5'-monophosphate dehydrogenase 2 (hIMPDH2), being an age-old target, has attracted attention recently for anticancer drug development. Mycophenolic acid (MPA), a well-known immunosuppressant drug, was used a lead structure to design and develop modestly potent and selective analogues. The steep structure-activity relationship (SAR) requirements of the lead molecule left little scope to synthesise newer analogues. Here, newer MPA amides were designed, synthesised and evaluated for hIMPDH2 inhibition and cellular efficacy in breast, prostate and glioblastoma cell lines. Few title compounds exhibited cellular activity profile better than MPA itself. The observed differences in the overall biological profile could be attributed to improved structural and physicochemical properties of the analogues over MPA. This is the first report of the activity of MPA derivatives in glioblastoma, the most aggressive brain cancer.
Keywords: MTT; Mycophenolic acid; anticancer agents; de novo purine synthesis; hIMPDH2 inhibitors.
Figures


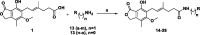
Similar articles
-
Discovery of novel human inosine 5'-monophosphate dehydrogenase 2 (hIMPDH2) inhibitors as potential anticancer agents.Eur J Med Chem. 2018 Oct 5;158:286-301. doi: 10.1016/j.ejmech.2018.09.016. Epub 2018 Sep 7. Eur J Med Chem. 2018. PMID: 30223117
-
Anticancer Properties of Amino Acid and Peptide Derivatives of Mycophenolic Acid.Anticancer Agents Med Chem. 2021;21(4):462-467. doi: 10.2174/1871520620666200516151456. Anticancer Agents Med Chem. 2021. PMID: 32416705
-
NAD-based inhibitors with anticancer potential.Bioorg Med Chem Lett. 2014 Jan 1;24(1):332-6. doi: 10.1016/j.bmcl.2013.11.005. Epub 2013 Nov 14. Bioorg Med Chem Lett. 2014. PMID: 24269162
-
The structure of inosine 5'-monophosphate dehydrogenase and the design of novel inhibitors.Immunopharmacology. 2000 May;47(2-3):163-84. doi: 10.1016/s0162-3109(00)00193-4. Immunopharmacology. 2000. PMID: 10878288 Review.
-
Synthesis of the inosine 5'-monophosphate dehydrogenase (IMPDH) inhibitors.J Enzyme Inhib Med Chem. 2015;30(4):550-63. doi: 10.3109/14756366.2014.951349. Epub 2014 Sep 8. J Enzyme Inhib Med Chem. 2015. PMID: 25198892 Review.
Cited by
-
Novel amides of mycophenolic acid and some heterocyclic derivatives as immunosuppressive agents.J Enzyme Inhib Med Chem. 2022 Dec;37(1):2725-2741. doi: 10.1080/14756366.2022.2127701. J Enzyme Inhib Med Chem. 2022. PMID: 36189734 Free PMC article.
-
De novo GTP synthesis is a metabolic vulnerability for the interception of brain metastases.Cell Rep Med. 2024 Oct 15;5(10):101755. doi: 10.1016/j.xcrm.2024.101755. Epub 2024 Oct 4. Cell Rep Med. 2024. PMID: 39366383 Free PMC article.
-
Quantitative analysis of differentially expressed proteins in psoriasis vulgaris using tandem mass tags and parallel reaction monitoring.Clin Proteomics. 2020 Aug 12;17:30. doi: 10.1186/s12014-020-09293-8. eCollection 2020. Clin Proteomics. 2020. PMID: 32817748 Free PMC article.
-
Purine-Metabolising Enzymes and Apoptosis in Cancer.Cancers (Basel). 2019 Sep 12;11(9):1354. doi: 10.3390/cancers11091354. Cancers (Basel). 2019. PMID: 31547393 Free PMC article. Review.
-
Approaches Towards Better Immunosuppressive Agents.Curr Top Med Chem. 2024;24(14):1230-1263. doi: 10.2174/0115680266292661240322072908. Curr Top Med Chem. 2024. PMID: 38561615 Review.
References
-
- Jackson RC, Weber G, Morris HP.. IMP dehydrogenase, an enzyme linked with proliferation and malignancy. Nature 1975;256:331–3. - PubMed
-
- Jayaram HN, Dion RL, Glazer RI, et al. . Initial studies on the mechanism of action of a new oncolytic thiazole nucleoside, 2-β-D-ribofuranosylthiazole-4-carboxamide (NSC 286193). Biochem Pharmacol 1982;31:2371–80. - PubMed
-
- Manzoli L, Billi AM, Gilmour RS, et al. . Phosphoinositide signaling in nuclei of friend cells: tiazofurin down-regulates phospholipase C beta 1. Cancer Res 1995;55:2978–80. - PubMed
-
- Mandanas BRA, Leibowitz DS, Gharehbaghi K, et al. . Role of p21 RAS in p210 bcr-abl transformation of murine myeloid cells. Blood 1993;82:1838–47. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
