Synthetic single domain antibodies for the conformational trapping of membrane proteins
- PMID: 29792401
- PMCID: PMC5967865
- DOI: 10.7554/eLife.34317
Synthetic single domain antibodies for the conformational trapping of membrane proteins
Abstract
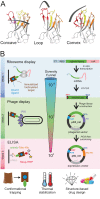
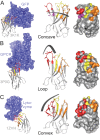
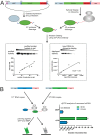
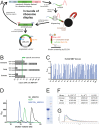
Mechanistic and structural studies of membrane proteins require their stabilization in specific conformations. Single domain antibodies are potent reagents for this purpose, but their generation relies on immunizations, which impedes selections in the presence of ligands typically needed to populate defined conformational states. To overcome this key limitation, we developed an in vitro selection platform based on synthetic single domain antibodies named sybodies. To target the limited hydrophilic surfaces of membrane proteins, we designed three sybody libraries that exhibit different shapes and moderate hydrophobicity of the randomized surface. A robust binder selection cascade combining ribosome and phage display enabled the generation of conformation-selective, high affinity sybodies against an ABC transporter and two previously intractable human SLC transporters, GlyT1 and ENT1. The platform does not require access to animal facilities and builds exclusively on commercially available reagents, thus enabling every lab to rapidly generate binders against challenging membrane proteins.
Keywords: E. coli; biochemistry; chemical biology; conformational trapping; in vitro selection; membrane protein; molecular biophysics; nanobody; phage display; ribosome display; structural biology.
© 2018, Zimmermann et al.
Conflict of interest statement
IZ, PE, CH, FA, SG, PS, DG, EG, MS No competing interests declared, PS Peter Stohler is affiliated with F. Hoffmann-La Roche Ltd. The author has no financial interests to declare. NB Nicolas Bocquet is affiliated with F. Hoffmann-La Roche Ltd. The author has no financial interests to declare. MH Melanie N Hug is affiliated with F. Hoffmann-La Roche Ltd. The author has no financial interests to declare. SH Sylwia Huber is affiliated with F. Hoffmann-La Roche Ltd. The author has no financial interests to declare. MS Martin Siegrist is affiliated with F. Hoffmann-La Roche Ltd. The author has no financial interests to declare. LH Lisa Hetemann is affiliated with F. Hoffmann-La Roche Ltd. The author has no financial interests to declare. JG Jennifer Gera is affiliated with F. Hoffmann-La Roche Ltd. The author has no financial interests to declare. RD Roger Dawson is affiliated with F. Hoffmann-La Roche Ltd. The author has no financial interests to declare.
Figures
















References
-
- Alberati D, Moreau JL, Lengyel J, Hauser N, Mory R, Borroni E, Pinard E, Knoflach F, Schlotterbeck G, Hainzl D, Wettstein JG. Glycine reuptake inhibitor RG1678: a pharmacologic characterization of an investigational agent for the treatment of schizophrenia. Neuropharmacology. 2012;62:1152–1161. doi: 10.1016/j.neuropharm.2011.11.008. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

