GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics
- PMID: 29792572
- PMCID: PMC5994659
- DOI: 10.1089/nat.2018.0736
GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics
Abstract
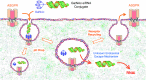
Short-interfering RNA (siRNA)-induced RNAi responses have great potential to treat a wide variety of human diseases from cancer to pandemic viral outbreaks to Parkinson's Disease. However, before siRNAs can become drugs, they must overcome a billion years of evolutionary defenses designed to keep invading RNAs on the outside cells from getting to the inside of cells. Not surprisingly, significant effort has been placed in developing a wide array of delivery technologies. Foremost of these has been the development of N-acetylgalactosamine (GalNAc) siRNA conjugates for delivery to liver. Tris-GalNAc binds to the Asialoglycoprotein receptor that is highly expressed on hepatocytes resulting in rapid endocytosis. While the exact mechanism of escape across the endosomal lipid bilayer membrane remains unknown, sufficient amounts of siRNAs enter the cytoplasm to induce robust, target selective RNAi responses in vivo. Multiple GalNAc-siRNA conjugate clinical trials, including two phase III trials, are currently underway by three biotech companies to treat a wide variety of diseases. GalNAc-siRNA conjugates are a simple solution to the siRNA delivery problem for liver hepatocytes and have shown the RNAi (and antisense oligonucleotide) field the path forward for targeting other tissue types.
Keywords: N-acetylgalactosamine; clinical trials; liver.
Conflict of interest statement
S.F.D. is a founder of Solstice Biologics. A.D.S. declares no competing financial interests exist.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

