Acid-sensing ion channels: dual function proteins for chemo-sensing and mechano-sensing
- PMID: 29793480
- PMCID: PMC5966886
- DOI: 10.1186/s12929-018-0448-y
Acid-sensing ion channels: dual function proteins for chemo-sensing and mechano-sensing
Abstract
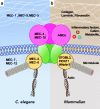
Background: Acid-sensing ion channels (ASICs) are a group of amiloride-sensitive ligand-gated ion channels belonging to the family of degenerin/epithelial sodium channels. ASICs are predominantly expressed in both the peripheral and central nervous system and have been characterized as potent proton sensors to detect extracellular acidification in the periphery and brain.
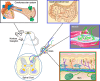
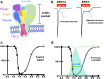
Main body: Here we review the recent studies focusing on the physiological roles of ASICs in the nervous system. As the major acid-sensing membrane proteins in the nervous system, ASICs detect tissue acidosis occurring at tissue injury, inflammation, ischemia, stroke, and tumors as well as fatiguing muscle to activate pain-sensing nerves in the periphery and transmit pain signals to the brain. Arachidonic acid and lysophosphocholine have been identified as endogenous non-proton ligands activating ASICs in a neutral pH environment. On the other hand, ASICs are found involved in the tether model mechanotransduction, in which the extracellular matrix and cytoplasmic cytoskeletons act like a gating-spring to tether the mechanically activated ion channels and thus transmit the stimulus force to the channels. Accordingly, accumulating evidence has shown ASICs play important roles in mechanotransduction of proprioceptors, mechanoreceptors and nociceptors to monitor the homoeostatic status of muscle contraction, blood volume, and blood pressure as well as pain stimuli.
Conclusion: Together, ASICs are dual-function proteins for both chemosensation and mechanosensation involved in monitoring physiological homoeostasis and pathological signals.
Keywords: ASIC; ASIC3; Mechanotransduction; Nociceptor; Pain; Proprioception.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Roles of ASICs in Nociception and Proprioception.Adv Exp Med Biol. 2018;1099:37-47. doi: 10.1007/978-981-13-1756-9_4. Adv Exp Med Biol. 2018. PMID: 30306513 Review.
-
Genetic exploration of roles of acid-sensing ion channel subtypes in neurosensory mechanotransduction including proprioception.Exp Physiol. 2024 Jan;109(1):66-80. doi: 10.1113/EP090762. Epub 2023 Jul 25. Exp Physiol. 2024. PMID: 37489658 Free PMC article. Review.
-
Acid-Sensing Ion Channels and Mechanosensation.Int J Mol Sci. 2021 May 1;22(9):4810. doi: 10.3390/ijms22094810. Int J Mol Sci. 2021. PMID: 34062742 Free PMC article. Review.
-
Neurosensory mechanotransduction through acid-sensing ion channels.J Cell Mol Med. 2013 Mar;17(3):337-49. doi: 10.1111/jcmm.12025. Epub 2013 Mar 14. J Cell Mol Med. 2013. PMID: 23490035 Free PMC article. Review.
-
ASIC3 channels in multimodal sensory perception.ACS Chem Neurosci. 2011 Jan 19;2(1):26-37. doi: 10.1021/cn100094b. Epub 2010 Nov 12. ACS Chem Neurosci. 2011. PMID: 22778854 Free PMC article. Review.
Cited by
-
Animal, Herb, and Microbial Toxins for Structural and Pharmacological Study of Acid-Sensing Ion Channels.Front Pharmacol. 2020 Jul 8;11:991. doi: 10.3389/fphar.2020.00991. eCollection 2020. Front Pharmacol. 2020. PMID: 32733241 Free PMC article. Review.
-
The Effects of Systemic and Local Acidosis on Insulin Resistance and Signaling.Int J Mol Sci. 2018 Dec 30;20(1):126. doi: 10.3390/ijms20010126. Int J Mol Sci. 2018. PMID: 30598026 Free PMC article. Review.
-
Sensing acidosis: nociception or sngception?J Biomed Sci. 2018 Nov 29;25(1):85. doi: 10.1186/s12929-018-0486-5. J Biomed Sci. 2018. PMID: 30486810 Free PMC article. Review.
-
Acid-Sensing Ion Channels' Immunoreactivity in Nerve Profiles and Glomus Cells of the Human Carotid Body.Int J Mol Sci. 2023 Dec 5;24(24):17161. doi: 10.3390/ijms242417161. Int J Mol Sci. 2023. PMID: 38138991 Free PMC article.
-
Neuronal Death Mechanisms and Therapeutic Strategy in Ischemic Stroke.Neurosci Bull. 2022 Oct;38(10):1229-1247. doi: 10.1007/s12264-022-00859-0. Epub 2022 May 5. Neurosci Bull. 2022. PMID: 35513682 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

