Integrating rare genetic variants into pharmacogenetic drug response predictions
- PMID: 29793534
- PMCID: PMC5968569
- DOI: 10.1186/s40246-018-0157-3
Integrating rare genetic variants into pharmacogenetic drug response predictions
Abstract
Background: Variability in genes implicated in drug pharmacokinetics or drug response can modulate treatment efficacy or predispose to adverse drug reactions. Besides common genetic polymorphisms, recent sequencing projects revealed a plethora of rare genetic variants in genes encoding proteins involved in drug metabolism, transport, and response.
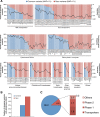
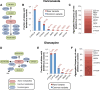
Results: To understand the global importance of rare pharmacogenetic gene variants, we mapped the variability in 208 pharmacogenes by analyzing exome sequencing data from 60,706 unrelated individuals and estimated the importance of rare and common genetic variants using a computational prediction framework optimized for pharmacogenetic assessments. Our analyses reveal that rare pharmacogenetic variants were strongly enriched in mutations predicted to cause functional alterations. For more than half of the pharmacogenes, rare variants account for the entire genetic variability. Each individual harbored on average a total of 40.6 putatively functional variants, rare variants accounting for 10.8% of these. Overall, the contribution of rare variants was found to be highly gene- and drug-specific. Using warfarin, simvastatin, voriconazole, olanzapine, and irinotecan as examples, we conclude that rare genetic variants likely account for a substantial part of the unexplained inter-individual differences in drug metabolism phenotypes.
Conclusions: Combined, our data reveal high gene and drug specificity in the contributions of rare variants. We provide a proof-of-concept on how this information can be utilized to pinpoint genes for which sequencing-based genotyping can add important information to predict drug response, which provides useful information for the design of clinical trials in drug development and the personalization of pharmacological treatment.
Keywords: ADME genes; Drug response; Genetic variability; Personalized medicine; Pharmacogenetics.
Conflict of interest statement
Ethics approval and consent to participate
In this study, only publically available, anonymized data released under the Fort Lauderdale Agreement was analyzed, and thus, separate ethical approval was not required.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

