A checklist for clinical trials in rare disease: obstacles and anticipatory actions-lessons learned from the FOR-DMD trial
- PMID: 29793540
- PMCID: PMC5968578
- DOI: 10.1186/s13063-018-2645-0
A checklist for clinical trials in rare disease: obstacles and anticipatory actions-lessons learned from the FOR-DMD trial
Abstract
Background: Trials in rare diseases have many challenges, among which are the need to set up multiple sites in different countries to achieve recruitment targets and the divergent landscape of clinical trial regulations in those countries. Over the past years, there have been initiatives to facilitate the process of international study set-up, but the fruits of these deliberations require time to be operationally in place. FOR-DMD (Finding the Optimum Steroid Regimen for Duchenne Muscular Dystrophy) is an academic-led clinical trial which aims to find the optimum steroid regimen for Duchenne muscular dystrophy, funded by the National Institutes of Health (NIH) for 5 years (July 2010 to June 2015), anticipating that all sites (40 across the USA, Canada, the UK, Germany and Italy) would be open to recruitment from July 2011. However, study start-up was significantly delayed and recruitment did not start until January 2013.
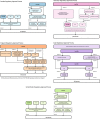
Method: The FOR-DMD study is used as an example to identify systematic problems in the set-up of international, multi-centre clinical trials. The full timeline of the FOR-DMD study, from funding approval to site activation, was collated and reviewed. Systematic issues were identified and grouped into (1) study set-up, e.g. drug procurement; (2) country set-up, e.g. competent authority applications; and (3) site set-up, e.g. contracts, to identify the main causes of delay and suggest areas where anticipatory action could overcome these obstacles in future studies.
Results: Time from the first contact to site activation across countries ranged from 6 to 24 months. Reasons of delay were universal (sponsor agreement, drug procurement, budgetary constraints), country specific (complexity and diversity of regulatory processes, indemnity requirements) and site specific (contracting and approvals). The main identified obstacles included (1) issues related to drug supply, (2) NIH requirements regarding contracting with non-US sites, (3) differing regulatory requirements in the five participating countries, (4) lack of national harmonisation with contracting and the requirement to negotiate terms and contract individually with each site and (5) diversity of languages needed for study materials. Additionally, as with many academic-led studies, the FOR-DMD study did not have access to the infrastructure and expertise that a contracted research organisation could provide, organisations often employed in pharmaceutical-sponsored studies. This delay impacted recruitment, challenged the clinical relevance of the study outcomes and potentially delayed the delivery of the best treatment to patients.
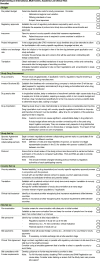
Conclusion: Based on the FOR-DMD experience, and as an interim solution, we have devised a checklist of steps to not only anticipate and minimise delays in academic international trial initiation but also identify obstacles that will require a concerted effort on the part of many stakeholders to mitigate.
Keywords: Academic-led clinical trial; Clinical trial; Clinical trial regulations; Duchenne muscular dystrophy; Rare disease.
Conflict of interest statement
Ethics approval and consent to participate
This manuscript does not directly contain human data; however, its topic is a clinical trial in which all patients were appropriately consented and ethics approval was granted from the University of Rochester Research Subjects Review Board (RSRB# 38795/#44449), as well as from the local ethics committees at each recruiting site.
Consent for publication
There was no specific informed consent sought for this publication because it does not contain any patient data. However, as part of the FOR-DMD clinical trial, full informed consent was obtained before any patient started the study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- International rare disease research consortium. Policies and guidelines. 2013. http://www.irdirc.org/wp-content/uploads/2017/10/IRDiRC_policies_24MayAp.... Accessed 8 May 2018.
-
- International rare disease research consortium. Visions and goals. http://www.irdirc.org/about-us/vision-goals/. Accessed May 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous