Herpes Simplex Virus 1 Tegument Protein VP22 Abrogates cGAS/STING-Mediated Antiviral Innate Immunity
- PMID: 29793952
- PMCID: PMC6052299
- DOI: 10.1128/JVI.00841-18
Herpes Simplex Virus 1 Tegument Protein VP22 Abrogates cGAS/STING-Mediated Antiviral Innate Immunity
Erratum in
-
Correction for Huang et al., "Herpes Simplex Virus 1 Tegument Protein VP22 Abrogates cGAS/STING-Mediated Antiviral Innate Immunity".J Virol. 2023 Nov 30;97(11):e0098223. doi: 10.1128/jvi.00982-23. Epub 2023 Oct 16. J Virol. 2023. PMID: 37843367 Free PMC article. No abstract available.
Abstract
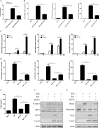
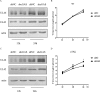
Cytosolic DNA arising from intracellular pathogens is sensed by cyclic GMP-AMP synthase (cGAS) and triggers a powerful innate immune response. However, herpes simplex virus 1 (HSV-1), a double-stranded DNA virus, has developed multiple mechanisms to attenuate host antiviral machinery and facilitate viral infection and replication. In the present study, we found that HSV-1 tegument protein VP22 acts as an inhibitor of cGAS/stimulator of interferon genes (cGAS/STING)-mediated production of interferon (IFN) and its downstream antiviral genes. Our results showed that ectopic expression of VP22 decreased cGAS/STING-mediated IFN-β promoter activation and IFN-β production. Infection with wild-type (WT) HSV-1, but not VP22-deficient virus (ΔVP22), inhibited immunostimulatory DNA (ISD)-induced activation of the IFN signaling pathway. Further study showed that VP22 interacted with cGAS and inhibited the enzymatic activity of cGAS. In addition, stable knockdown of cGAS facilitated the replication of ΔVP22 virus but not the WT. In summary, our findings indicate that HSV-1 VP22 acts as an antagonist of IFN signaling to persistently evade host innate antiviral responses.IMPORTANCE cGAS is very important for host defense against viral infection, and many viruses have evolved ways to target cGAS and successfully evade the attack by the immune system of their susceptible host. This study demonstrated that HSV-1 tegument protein VP22 counteracts the cGAS/STING-mediated DNA-sensing antiviral innate immunity signaling pathway by inhibiting the enzymatic activity of cGAS. The findings in this study will expand our understanding of the interaction between HSV-1 replication and the host DNA-sensing signaling pathway.
Keywords: DNA sensing; HSV-1; VP22; cGAS.
Copyright © 2018 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

