Effects of mindfulness training programmes delivered by a self-directed mobile app and by telephone compared with an education programme for survivors of critical illness: a pilot randomised clinical trial
- PMID: 29793970
- PMCID: PMC6460929
- DOI: 10.1136/thoraxjnl-2017-211264
Effects of mindfulness training programmes delivered by a self-directed mobile app and by telephone compared with an education programme for survivors of critical illness: a pilot randomised clinical trial
Abstract
Background: Patients who are sick enough to be admitted to an intensive care unit (ICU) commonly experience symptoms of psychological distress after discharge, yet few effective therapies have been applied to meet their needs.
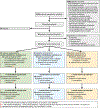
Methods: Pilot randomised clinical trial with 3-month follow-up conducted at two academic medical centres. Adult (≥18 years) ICU patients treated for cardiorespiratory failure were randomised after discharge home to 1 of 3 month-long interventions: a self-directed mobile app-based mindfulness programme; a therapist-led telephone-based mindfulness programme; or a web-based critical illness education programme.
Results: Among 80 patients allocated to mobile mindfulness (n=31), telephone mindfulness (n=31) or education (n=18), 66 (83%) completed the study. For the primary outcomes, target benchmarks were exceeded by observed rates for all participants for feasibility (consent 74%, randomisation 91%, retention 83%), acceptability (mean Client Satisfaction Questionnaire 27.6 (SD 3.8)) and usability (mean Systems Usability Score 89.1 (SD 11.5)). For secondary outcomes, mean values (and 95% CIs) reflected clinically significant group-based changes on the Patient Health Questionnaire depression scale (mobile (-4.8 (-6.6, -2.9)), telephone (-3.9 (-5.6, -2.2)), education (-3.0 (-5.3, 0.8)); the Generalized Anxiety Disorder scale (mobile -2.1 (-3.7, -0.5), telephone -1.6 (-3.0, -0.1), education -0.6 (-2.5, 1.3)); the Post-Traumatic Stress Scale (mobile -2.6 (-6.3, 1.2), telephone -2.2 (-5.6, 1.2), education -3.5 (-8.0, 1.0)); and the Patient Health Questionnaire physical symptom scale (mobile -5.3 (-7.0, -3.7), telephone -3.7 (-5.2, 2.2), education -4.8 (-6.8, 2.7)).
Conclusions: Among ICU patients, a mobile mindfulness app initiated after hospital discharge demonstrated evidence of feasibility, acceptability and usability and had a similar impact on psychological distress and physical symptoms as a therapist-led programme. A larger trial is warranted to formally test the efficacy of this approach.
Trial registration number: Results, NCT02701361.
Keywords: critical illness; depression; mind and body; mindfulness; mobile app; psychological distress.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2019. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures

Comment in
-
Process evaluation and the development of behavioural interventions to improve psychological distress among survivors of critical illness.Thorax. 2019 Jan;74(1):7-10. doi: 10.1136/thoraxjnl-2018-211989. Epub 2018 Oct 18. Thorax. 2019. PMID: 30337416 Free PMC article. No abstract available.
References
-
- Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003;348:683–693. - PubMed
-
- Wunsch H, Christiansen CF, Johansen MB, et al. Psychiatric diagnoses and psychoactive medication use among nonsurgical critically ill patients receiving mechanical ventilation. JAMA 2014;311:1133–42. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
