Cortical Oscillatory Mechanisms Supporting the Control of Human Social-Emotional Actions
- PMID: 29793973
- PMCID: PMC6595981
- DOI: 10.1523/JNEUROSCI.3382-17.2018
Cortical Oscillatory Mechanisms Supporting the Control of Human Social-Emotional Actions
Abstract
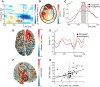
The human anterior prefrontal cortex (aPFC) is involved in regulating social-emotional behavior, presumably by modulating effective connectivity with downstream parietal, limbic, and motor cortices. Regulating that connectivity might rely on theta-band oscillations (4-8 Hz), a brain rhythm known to create overlapping periods of excitability between distant regions by temporally releasing neurons from inhibition. Here, we used MEG to understand how aPFC theta-band oscillations implement control over prepotent social-emotional behaviors; that is, the control over automatically elicited approach and avoidance actions. Forty human male participants performed a social approach-avoidance task in which they approached or avoided visually displayed emotional faces (happy or angry) by pulling or pushing a joystick. Approaching angry and avoiding happy faces (incongruent condition) requires rapid application of cognitive control to override prepotent habitual action tendencies to approach appetitive and to avoid aversive situations. In the time window before response delivery, trial-by-trial variations in aPFC theta-band power (6 Hz) predicted reaction time increases during emotional control and were inversely related to beta-band power (14-22 Hz) over parietofrontal cortex. In sensorimotor areas contralateral to the moving hand, premovement gamma-band rhythms (60-90 Hz) were stronger during incongruent than congruent trials, with power increases phase locked to peaks of the aPFC theta-band oscillations. These findings define a mechanistic relation between cortical areas involved in implementing rapid control over human social-emotional behavior. The aPFC may bias neural processing toward rule-driven actions and away from automatic emotional tendencies by coordinating tonic disinhibition and phasic enhancement of parietofrontal circuits involved in action selection.SIGNIFICANCE STATEMENT Being able to control social-emotional behavior is crucial for successful participation in society, as is illustrated by the severe social and occupational difficulties experienced by people suffering from social motivational disorders such as social anxiety. In this study, we show that theta-band oscillations in the anterior prefrontal cortex (aPFC), which are thought to provide temporal organization for neural firing during communication between distant brain areas, facilitate this control by linking aPFC to parietofrontal beta-band and sensorimotor gamma-band oscillations involved in action selection. These results contribute to a mechanistic understanding of cognitive control over automatic social-emotional action and point to frontal theta-band oscillations as a possible target of rhythmic neurostimulation techniques during treatment for social anxiety.
Keywords: MEG; approach–avoidance; frontal pole; phase-amplitude coupling; social–emotional control; theta-band oscillations.
Copyright © 2018 the authors 0270-6474/18/385739-11$15.00/0.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
