ZMPSTE24 missense mutations that cause progeroid diseases decrease prelamin A cleavage activity and/or protein stability
- PMID: 29794150
- PMCID: PMC6078402
- DOI: 10.1242/dmm.033670
ZMPSTE24 missense mutations that cause progeroid diseases decrease prelamin A cleavage activity and/or protein stability
Abstract
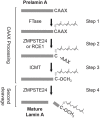
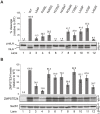
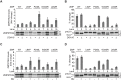
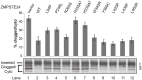
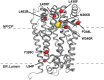
The human zinc metalloprotease ZMPSTE24 is an integral membrane protein crucial for the final step in the biogenesis of the nuclear scaffold protein lamin A, encoded by LMNA After farnesylation and carboxyl methylation of its C-terminal CAAX motif, the lamin A precursor (prelamin A) undergoes proteolytic removal of its modified C-terminal 15 amino acids by ZMPSTE24. Mutations in LMNA or ZMPSTE24 that impede this prelamin A cleavage step cause the premature aging disease Hutchinson-Gilford progeria syndrome (HGPS), and the related progeroid disorders mandibuloacral dysplasia type B (MAD-B) and restrictive dermopathy (RD). Here, we report the development of a 'humanized yeast system' to assay ZMPSTE24-dependent cleavage of prelamin A and examine the eight known disease-associated ZMPSTE24 missense mutations. All mutations show diminished prelamin A processing and fall into three classes, with defects in activity, protein stability or both. Notably, some ZMPSTE24 mutants can be rescued by deleting the E3 ubiquitin ligase Doa10, involved in endoplasmic reticulum (ER)-associated degradation of misfolded membrane proteins, or by treatment with the proteasome inhibitor bortezomib. This finding may have important therapeutic implications for some patients. We also show that ZMPSTE24-mediated prelamin A cleavage can be uncoupled from the recently discovered role of ZMPSTE24 in clearance of ER membrane translocon-clogged substrates. Together with the crystal structure of ZMPSTE24, this humanized yeast system can guide structure-function studies to uncover mechanisms of prelamin A cleavage, translocon unclogging, and membrane protein folding and stability.
Keywords: Lamin A processing; Progeria disease; Saccharomyces cerevisiae; Ubiquitin-proteasome system.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

