The high-affinity calcium sensor synaptotagmin-7 serves multiple roles in regulated exocytosis
- PMID: 29794152
- PMCID: PMC5987875
- DOI: 10.1085/jgp.201711944
The high-affinity calcium sensor synaptotagmin-7 serves multiple roles in regulated exocytosis
Abstract
Synaptotagmin (Syt) proteins comprise a 17-member family, many of which trigger exocytosis in response to calcium. Historically, most studies have focused on the isoform Syt-1, which serves as the primary calcium sensor in synchronous neurotransmitter release. Recently, Syt-7 has become a topic of broad interest because of its extreme calcium sensitivity and diversity of roles in a wide range of cell types. Here, we review the known and emerging roles of Syt-7 in various contexts and stress the importance of its actions. Unique functions of Syt-7 are discussed in light of recent imaging, electrophysiological, and computational studies. Particular emphasis is placed on Syt-7-dependent regulation of synaptic transmission and neuroendocrine cell secretion. Finally, based on biochemical and structural data, we propose a mechanism to link Syt-7's role in membrane fusion with its role in subsequent fusion pore expansion via strong calcium-dependent phospholipid binding.
© 2018 MacDougall et al.
Figures
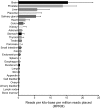
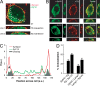





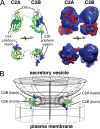
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

