The Aspergillus nidulans Pyruvate Dehydrogenase Kinases Are Essential To Integrate Carbon Source Metabolism
- PMID: 29794164
- PMCID: PMC6027865
- DOI: 10.1534/g3.118.200411
The Aspergillus nidulans Pyruvate Dehydrogenase Kinases Are Essential To Integrate Carbon Source Metabolism
Abstract
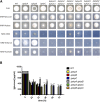
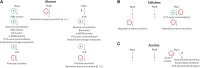
The pyruvate dehydrogenase complex (PDH), that converts pyruvate to acetyl-coA, is regulated by pyruvate dehydrogenase kinases (PDHK) and phosphatases (PDHP) that have been shown to be important for morphology, pathogenicity and carbon source utilization in different fungal species. The aim of this study was to investigate the role played by the three PDHKs PkpA, PkpB and PkpC in carbon source utilization in the reference filamentous fungus Aspergillus nidulans, in order to unravel regulatory mechanisms which could prove useful for fungal biotechnological and biomedical applications. PkpA and PkpB were shown to be mitochondrial whereas PkpC localized to the mitochondria in a carbon source-dependent manner. Only PkpA was shown to regulate PDH activity. In the presence of glucose, deletion of pkpA and pkpC resulted in reduced glucose utilization, which affected carbon catabolite repression (CCR) and hydrolytic enzyme secretion, due to de-regulated glycolysis and TCA cycle enzyme activities. Furthermore, PkpC was shown to be required for the correct metabolic utilization of cellulose and acetate. PkpC negatively regulated the activity of the glyoxylate cycle enzyme isocitrate lyase (ICL), required for acetate metabolism. In summary, this study identified PDHKs important for the regulation of central carbon metabolism in the presence of different carbon sources, with effects on the secretion of biotechnologically important enzymes and carbon source-related growth. This work demonstrates how central carbon metabolism can affect a variety of fungal traits and lays a basis for further investigation into these characteristics with potential interest for different applications.
Keywords: Aspergillus nidulans; carbon catabolite repression; carbon source utilization and regulation; pyruvate dehydrogenase kinases.
Copyright © 2018 Ries et al.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
