Use of trastuzumab as an adjuvant/neoadjuvant therapy in patients with HER2-positive breast cancer in China: The Nvwa study
- PMID: 29794725
- PMCID: PMC6393039
- DOI: 10.1097/MD.0000000000010350
Use of trastuzumab as an adjuvant/neoadjuvant therapy in patients with HER2-positive breast cancer in China: The Nvwa study
Abstract
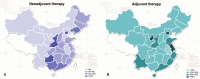
The aim of this study was to understand current trends in trastuzumab use in China as a neoadjuvant/adjuvant therapy for human epidermal growth factor receptor-2 positive (HER2+) breast cancer and identify factors influencing trastuzumab use.This was a retrospective, multicenter, cross-sectional study of patients diagnosed with HER2+ breast cancer (stage I-III), between July 2013 and June 2014, at 155 hospitals in 29 provinces/cities in China. Demographic and clinical data, including tumor characteristics and details of adjuvant/neoadjuvant therapies used, were collected. Data analysis included univariate analysis, multivariate logistic regression, and subgroup analyses.Of 4994 HER2+ patients (mean age 51.1 ± 9.9 years) included, only 29.8% received trastuzumab, with 30.5% in adjuvant therapy and 18.3% in neoadjuvant therapy. The highest rates of adjuvant trastuzumab were in Beijing (59.3%), Jiangsu (57.1%), and Ningxia (50.0%), while those of neoadjuvant trastuzumab were in Guangdong (24.8%), Beijing (14.1%), and Zhejiang (10.7%). Multivariate regression results revealed that factors associated with trastuzumab use were medical insurance cover for trastuzumab, residing locally to the hospital, more lymph node involvement, and more advanced tumor stage. Subgroup analysis revealed that patients receiving neoadjuvant therapy were likely to be younger, premenopausal and non-local, and had lymph node metastases, more advanced tumor, and progesterone receptor positive tumor.Trastuzumab use in patients with HER2+ breast cancer is relatively low in China, especially for neoadjuvant therapy. Insurance coverage seems to be the most correlated factor that influences the use of trastuzumab in Chinese patients with HER2+ breast cancer.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

