Multiple, Independent T Cell Lymphomas Arising in an Experimentally FIV-Infected Cat during the Terminal Stage of Infection
- PMID: 29794987
- PMCID: PMC6024646
- DOI: 10.3390/v10060280
Multiple, Independent T Cell Lymphomas Arising in an Experimentally FIV-Infected Cat during the Terminal Stage of Infection
Abstract
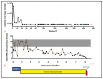
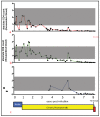
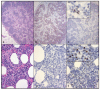
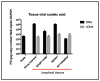
Our laboratory has serially reported on the virologic and immunopathologic features of a cohort of experimental feline immunodeficiency virus (FIV)-infected cats for more than eight years. At 8.09 years post infection (PI), one of these animals entered the terminal stage of infection, characterized by undulating hyperthermia, progressive anorexia, weight loss, and pancytopenia; the animal was not responsive to therapeutic interventions, necessitating euthanasia six weeks later (8.20 years PI). Subsequent analyses indicated that neoplastic lymphocytes infiltrated multiple cervical lymph nodes and a band-like region of the mucosal lamina propria within a segment of the intestine. Immunohistochemistry and T cell clonality testing determined that the nodal and intestinal lesions were independently arising from CD3 T cell lymphomas. In-situ RNA hybridization studies indicated that diffuse neoplastic lymphocytes from the cervical lymph node contained abundant viral nucleic acid, while viral nucleic acid was not detectable in lymphocytes from the intestinal lymphoma lesion. The proviral long terminal repeat (LTR) was amplified and sequenced from multiple anatomic sites, and a common clone containing a single nucleotide polymorphism was determined to be defective in response to phorbol myristate acetate (PMA)-mediated promoter activation in a reporter gene assay. This assay revealed a previously unidentified PMA response element within the FIV U3 region 3' to the TATA box. The possible implications of these results on FIV-lymphoma pathogenesis are discussed.
Keywords: FAIDS (feline AIDS); FIV (feline immunodeficiency virus); cat; immunopathology; lymphoma.
Conflict of interest statement
The authors declare no conflict of interest. The sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Figures

Similar articles
-
Viral Reservoirs in Lymph Nodes of FIV-Infected Progressor and Long-Term Non-Progressor Cats during the Asymptomatic Phase.PLoS One. 2016 Jan 7;11(1):e0146285. doi: 10.1371/journal.pone.0146285. eCollection 2016. PLoS One. 2016. PMID: 26741651 Free PMC article.
-
Regulation of gene expression directed by the long terminal repeat of the feline immunodeficiency virus.Virology. 1992 Mar;187(1):165-77. doi: 10.1016/0042-6822(92)90305-9. Virology. 1992. PMID: 1310554
-
Peripheral immunophenotype and viral promoter variants during the asymptomatic phase of feline immunodeficiency virus infection.Virus Res. 2014 Jan 22;179:34-43. doi: 10.1016/j.virusres.2013.11.017. Epub 2013 Nov 28. Virus Res. 2014. PMID: 24291288 Free PMC article.
-
Vaginal and rectal infection of cats with feline immunodeficiency virus.Vet Microbiol. 1996 Aug;51(3-4):217-27. doi: 10.1016/0378-1135(96)00038-7. Vet Microbiol. 1996. PMID: 8870185
-
Characterization of a highly pathogenic molecular clone of feline immunodeficiency virus clade C.J Virol. 2004 Sep;78(17):8971-82. doi: 10.1128/JVI.78.17.8971-8982.2004. J Virol. 2004. PMID: 15308694 Free PMC article.
Cited by
-
Clonality testing in the lymph nodes from dogs with lymphadenomegaly due to Leishmania infantum infection.PLoS One. 2019 Dec 16;14(12):e0226336. doi: 10.1371/journal.pone.0226336. eCollection 2019. PLoS One. 2019. PMID: 31841533 Free PMC article.
-
Exploring the link between viruses and cancer in companion animals: a comprehensive and comparative analysis.Infect Agent Cancer. 2023 Jun 29;18(1):40. doi: 10.1186/s13027-023-00518-7. Infect Agent Cancer. 2023. PMID: 37386451 Free PMC article. Review.
-
Infectious Causes of Neoplasia in the Domestic Cat.Vet Sci. 2022 Aug 30;9(9):467. doi: 10.3390/vetsci9090467. Vet Sci. 2022. PMID: 36136683 Free PMC article. Review.
-
The Late Asymptomatic and Terminal Immunodeficiency Phases in Experimentally FIV-Infected Cats-A Long-Term Study.Viruses. 2023 Aug 21;15(8):1775. doi: 10.3390/v15081775. Viruses. 2023. PMID: 37632117 Free PMC article.
-
An RNA-Directed Gene Editing Strategy for Attenuating the Infectious Potential of Feline Immunodeficiency Virus-Infected Cells: A Proof of Concept.Viruses. 2020 May 5;12(5):511. doi: 10.3390/v12050511. Viruses. 2020. PMID: 32380756 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

