Actinomycetes, an Inexhaustible Source of Naturally Occurring Antibiotics
- PMID: 29795019
- PMCID: PMC6022875
- DOI: 10.3390/antibiotics7020045
Actinomycetes, an Inexhaustible Source of Naturally Occurring Antibiotics
Erratum in
-
Correction: Yōko, T., et al. Actinomycetes, an Inexhaustible Source of Naturally Occurring Antibiotics. Antibiotics 2018, 7, 45.Antibiotics (Basel). 2018 Aug 14;7(3):74. doi: 10.3390/antibiotics7030074. Antibiotics (Basel). 2018. PMID: 30110972 Free PMC article.
Abstract
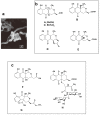
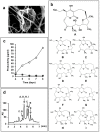
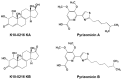
Global public health faces a desperate situation, due to the lack of effective antibiotics. Coordinated steps need to be taken, worldwide, to rectify this situation and protect the advances in modern medicine made over the last 100 years. Work at Japan's Kitasato Institute has been in the vanguard of many such advances, and work is being proactively tailored to promote the discovery of urgently needed antimicrobials. Efforts are being concentrated on actinomycetes, the proven source of most modern antibiotics. We devised a novel physicochemical screening mechanism, whereby simple physico-chemical properties, in conjunction with related detection methods, such as LC/MS, LC/UV, and polarity, could be used to identify or predict new compounds in a culture broth, simply by comparing results with existing databases. New compounds are isolated, purified, and their structure determined before being tested for any bioactivity. We used lyophilized actinomycete strains from the Kitasato Microbial Library, most more than 35 years old, and found 330 strains were producers of useful bioactive substances. We also tested organisms found in fresh samples collected in the complex environments from around plant roots, as well as from sediments of mangrove forests and oceans, resulting in the discovery of 36 novel compounds from 11 actinomycete strains. A compound, designated iminimycin, containing an iminium ion in the structure was discovered from the culture broth of Streptomyces griseus OS-3601, which had been stored for a long time as a streptomycin-producing strain. This represented the first iminium ion discovery in actinomycetes. Compounds with a cyclopentadecane skeleton containing 5,6-dihydro-4-hydroxyl-2-pyrone ring and tetrahydrofuran ring, designated mangromicins, were isolated from the culture broth of Lechevalieria aerocolonigenes K10-0216 obtained from sediment in a mangrove forest. These structures are extremely unique among natural compounds. From the same culture broth, new steroid compounds, named K10-0216 KA and KB, and other new compounds having a thiazole and a pyridine ring, named pyrizomicin A and B, were discovered. New substances can be found from actinomycetes that have been exhaustively studied. Novel compounds with different skeletons can be found from a single broth of one strain. The sought after new antibiotics will arise from continued exploitation of the actinomycetes, especially rare actinomycetes. Work on new organisms and samples should be augmented by re-examination of known actinomycetes already in storage. New research should also be carried out on the manipulation of culture media, thereby stimulating actinomycete strains to produce novel chemicals. The establishment of wide-ranging international research collaborations will facilitate and expedite the efficient and timely discovery and provision of bioactive compounds to help maintain and promote advances in global public health.
Keywords: actinomycetes; biological activity; novel compounds; physical and chemical properties; physicochemical screening; secondary metabolites; structural diversity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Dimasi J.A., Grabowski G.H., Hansen R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016;47:20–33. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

