Saracatinib Inhibits Middle East Respiratory Syndrome-Coronavirus Replication In Vitro
- PMID: 29795047
- PMCID: PMC6024778
- DOI: 10.3390/v10060283
Saracatinib Inhibits Middle East Respiratory Syndrome-Coronavirus Replication In Vitro
Abstract
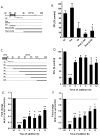
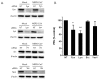
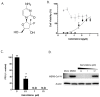
The Middle East respiratory syndrome-coronavirus (MERS-CoV), first identified in Saudi Arabia, is an emerging zoonotic pathogen that causes severe acute respiratory illness in humans with a high fatality rate. Since its emergence, MERS-CoV continues to spread to countries outside of the Arabian Peninsula and gives rise to sporadic human infections following the entry of infected individuals to other countries, which can precipitate outbreaks similar to the one that occurred in South Korea in 2015. Current therapeutics against MERS-CoV infection have primarily been adapted from previous drugs used for the treatment of severe acute respiratory syndrome. In search of new potential drug candidates, we screened a library composed of 2334 clinically approved drugs and pharmacologically active compounds. The drug saracatinib, a potent inhibitor of Src-family of tyrosine kinases (SFK), was identified as an inhibitor of MERS-CoV replication in vitro. Our results suggest that saracatinib potently inhibits MERS-CoV at the early stages of the viral life cycle in Huh-7 cells, possibly through the suppression of SFK signaling pathways. Furthermore, saracatinib exhibited a synergistic effect with gemcitabine, an anticancer drug with antiviral activity against several RNA viruses. These data indicate that saracatinib alone or in combination with gemcitabine can provide a new therapeutic option for the treatment of MERS-CoV infection.
Keywords: MERS-CoV; Middle East Respiratory Syndrome; Src-family kinase inhibitor; gemcitabine; saracatinib.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

