Burst activation of dopamine neurons produces prolonged post-burst availability of actively released dopamine
- PMID: 29795245
- PMCID: PMC6098082
- DOI: 10.1038/s41386-018-0088-7
Burst activation of dopamine neurons produces prolonged post-burst availability of actively released dopamine
Abstract
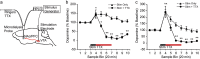
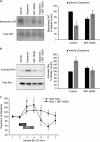
Both phasic and tonic modes of neurotransmission are implicated in critical functions assigned to dopamine. In learning, for example, sub-second phasic responses of ventral tegmental area (VTA) dopamine neurons to salient events serve as teaching signals, but learning is also interrupted by dopamine antagonists administered minutes after training. Our findings bridge the multiple timescales of dopamine neurotransmission by demonstrating that burst stimulation of VTA dopamine neurons produces a prolonged post-burst increase (>20 min) of extracellular dopamine in nucleus accumbens and prefrontal cortex. This elevation is not due to spillover from the stimulation surge but depends on impulse flow-mediated dopamine release. We identified Rho-mediated internalization of dopamine transporter as a mechanism responsible for prolonged availability of actively released dopamine. Thus, a critical consequence of burst activity of dopamine neurons may be post-burst sustained elevation of extracellular dopamine in terminal regions via an intracellular mechanism that promotes dopamine transporter internalization. These results demonstrate that phasic and tonic dopamine neurotransmission can be a continuum and may explain why both modes of signaling are critical for motivational and cognitive functions associated with dopamine.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

