Immunoglobulin light chain amyloidosis diagnosis and treatment algorithm 2018
- PMID: 29795248
- PMCID: PMC5966459
- DOI: 10.1038/s41408-018-0080-9
Immunoglobulin light chain amyloidosis diagnosis and treatment algorithm 2018
Abstract
Immunoglobulin light chain amyloidosis (AL) should be considered in any patient that presents to a cancer care provider with nephrotic range proteinuria, heart failure with preserved ejection fraction, non-diabetic peripheral neuropathy, unexplained hepatomegaly or diarrhea. More importantly, patients being monitored for smoldering multiple myeloma and a monoclonal gammopathy of undetermined significance (MGUS) are at risk for developing AL amyloidosis. MGUS and myeloma patients that have atypical features, including unexplained weight loss; lower extremity edema, early satiety, and dyspnea on exertion should be considered at risk for light chain amyloidosis. Overlooking the diagnosis of light chain amyloidosis leading to therapy delay is common, and it represents an error of diagnostic consideration. Algorithms will be provided on how to evaluate patients with suspected AL amyloid as well as how to manage patients referred from other medical specialties with biopsy-proven amyloid. An organized stepwise approach to the treatment of patients with light chain amyloidosis, including established and investigational therapies, will be reviewed.
Conflict of interest statement
M.A.G. reports personal fees from Ionis, personal fees from Alnylym, personal fees from Prothena, personal fees from Celgene, personal fees from Janssen, grants and personal fees from Spectrum, personal fees from Annexon, personal fees from Appellis, personal fees from Amgen, personal fees from Medscape, personal fees from Physicians Education Resource, personal fees from Abbvie, personal fees from Research to Practice, from Teva, outside the submitted work.
Figures



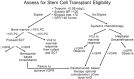
References
-
- Kyle RA, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79:1817–1822. - PubMed
-
- Quock TP, Yan JT, Chang E, Guthrie SD, Broder MS. Epidemiology of AL amyloidosis in a US commercially insured population. Blood. 2017;130(Suppl 1):5335.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

