Cholinergic activity is essential for maintaining the anterograde transport of Choline Acetyltransferase in Drosophila
- PMID: 29795337
- PMCID: PMC5966444
- DOI: 10.1038/s41598-018-26176-z
Cholinergic activity is essential for maintaining the anterograde transport of Choline Acetyltransferase in Drosophila
Abstract
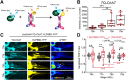
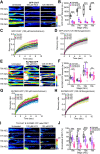
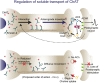
Cholinergic activity is essential for cognitive functions and neuronal homeostasis. Choline Acetyltransferase (ChAT), a soluble protein that synthesizes acetylcholine at the presynaptic compartment, is transported in bulk in the axons by the heterotrimeric Kinesin-2 motor. Axonal transport of soluble proteins is described as a constitutive process assisted by occasional, non-specific interactions with moving vesicles and motor proteins. Here, we report that an increase in the influx of Kinesin-2 motor and association between ChAT and the motor during a specific developmental period enhances the axonal entry, as well as the anterograde flow of the protein, in the sensory neurons of intact Drosophila nervous system. Loss of cholinergic activity due to Hemicholinium and Bungarotoxin treatments, respectively, disrupts the interaction between ChAT and Kinesin-2 in the axon, and the episodic enhancement of axonal influx of the protein. Altogether, these observations highlight a phenomenon of synaptic activity-dependent, feedback regulation of a soluble protein transport in vivo, which could potentially define the quantum of its pre-synaptic influx.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

