Reciprocal signalling by Notch-Collagen V-CALCR retains muscle stem cells in their niche
- PMID: 29795344
- PMCID: PMC5985950
- DOI: 10.1038/s41586-018-0144-9
Reciprocal signalling by Notch-Collagen V-CALCR retains muscle stem cells in their niche
Erratum in
-
Author Correction: Reciprocal signalling by Notch-Collagen V-CALCR retains muscle stem cells in their niche.Nature. 2025 Nov;647(8091):E4. doi: 10.1038/s41586-025-09787-1. Nature. 2025. PMID: 41168534 No abstract available.
Abstract
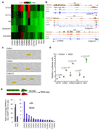
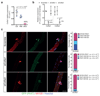
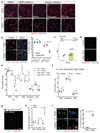
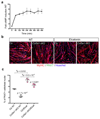
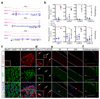
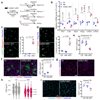
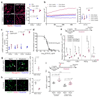
The cell microenvironment, which is critical for stem cell maintenance, contains both cellular and non-cellular components, including secreted growth factors and the extracellular matrix1-3. Although Notch and other signalling pathways have previously been reported to regulate quiescence of stem cells4-9, the composition and source of molecules that maintain the stem cell niche remain largely unknown. Here we show that adult muscle satellite (stem) cells in mice produce extracellular matrix collagens to maintain quiescence in a cell-autonomous manner. Using chromatin immunoprecipitation followed by sequencing, we identified NOTCH1/RBPJ-bound regulatory elements adjacent to specific collagen genes, the expression of which is deregulated in Notch-mutant mice. Moreover, we show that Collagen V (COLV) produced by satellite cells is a critical component of the quiescent niche, as depletion of COLV by conditional deletion of the Col5a1 gene leads to anomalous cell cycle entry and gradual diminution of the stem cell pool. Notably, the interaction of COLV with satellite cells is mediated by the Calcitonin receptor, for which COLV acts as a surrogate local ligand. Systemic administration of a calcitonin derivative is sufficient to rescue the quiescence and self-renewal defects found in COLV-null satellite cells. This study reveals a Notch-COLV-Calcitonin receptor signalling cascade that maintains satellite cells in a quiescent state in a cell-autonomous fashion, and raises the possibility that similar reciprocal mechanisms act in diverse stem cell populations.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Comment in
-
A self-made quiescent niche.Nat Rev Mol Cell Biol. 2018 Jul;19(7):416-417. doi: 10.1038/s41580-018-0031-0. Nat Rev Mol Cell Biol. 2018. PMID: 29875372 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

