An antibacterial platform based on capacitive carbon-doped TiO2 nanotubes after direct or alternating current charging
- PMID: 29795383
- PMCID: PMC5967314
- DOI: 10.1038/s41467-018-04317-2
An antibacterial platform based on capacitive carbon-doped TiO2 nanotubes after direct or alternating current charging
Abstract
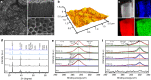
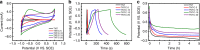
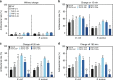
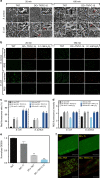
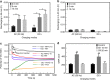
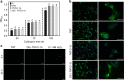
Electrical interactions between bacteria and the environment are delicate and essential. In this study, an external electrical current is applied to capacitive titania nanotubes doped with carbon (TNT-C) to evaluate the effects on bacteria killing and the underlying mechanism is investigated. When TNT-C is charged, post-charging antibacterial effects proportional to the capacitance are observed. This capacitance-based antibacterial system works well with both direct and alternating current (DC, AC) and the higher discharging capacity in the positive DC (DC+) group leads to better antibacterial performance. Extracellular electron transfer observed during early contact contributes to the surface-dependent post-charging antibacterial process. Physiologically, the electrical interaction deforms the bacteria morphology and elevates the intracellular reactive oxygen species level without impairing the growth of osteoblasts. Our finding spurs the design of light-independent antibacterial materials and provides insights into the use of electricity to modify biomaterials to complement other bacteria killing measures such as light irradiation.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

