Design of optimal nonlinear network controllers for Alzheimer's disease
- PMID: 29795548
- PMCID: PMC5967700
- DOI: 10.1371/journal.pcbi.1006136
Design of optimal nonlinear network controllers for Alzheimer's disease
Abstract
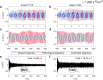
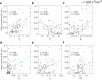
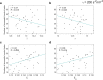
Brain stimulation can modulate the activity of neural circuits impaired by Alzheimer's disease (AD), having promising clinical benefit. However, all individuals with the same condition currently receive identical brain stimulation, with limited theoretical basis for this generic approach. In this study, we introduce a control theory framework for obtaining exogenous signals that revert pathological electroencephalographic activity in AD at a minimal energetic cost, while reflecting patients' biological variability. We used anatomical networks obtained from diffusion magnetic resonance images acquired by the Alzheimer's Disease Neuroimaging Initiative (ADNI) as mediators for the interaction between Duffing oscillators. The nonlinear nature of the brain dynamics is preserved, given that we extend the so-called state-dependent Riccati equation control to reflect the stimulation objective in the high-dimensional neural system. By considering nonlinearities in our model, we identified regions for which control inputs fail to correct abnormal activity. There are changes to the way stimulated regions are ranked in terms of the energetic cost of controlling the entire network, from a linear to a nonlinear approach. We also found that limbic system and basal ganglia structures constitute the top target locations for stimulation in AD. Patients with highly integrated anatomical networks-namely, networks having low average shortest path length, high global efficiency-are the most suitable candidates for the propagation of stimuli and consequent success on the control task. Other diseases associated with alterations in brain dynamics and the self-control mechanisms of the brain can be addressed through our framework.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Perez JM, Evans AC, Initiative TADN. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun [Internet]. 2016;7(May):11934 Available from: 10.1038/ncomms11934%255Cnhttp://10.1038/ncomms11934 - DOI - PMC - PubMed
-
- Iturria-Medina Y, Carbonell FM, Sotero RC, Chouinard-Decorte F, Evans AC. Multifactorial causal model of brain (dis)organization and therapeutic intervention: Application to Alzheimer’s disease. Neuroimage [Internet]. 2017;152(February):60–77. Available from: 10.1016/j.neuroimage.2017.02.058 - DOI - PubMed
-
- Hsu W-Y, Ku Y, Zanto TP, Gazzaley A. Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer’s disease: a systematic review and meta-analysis. Neurobiol Aging [Internet]. 2015;36(8):2348–59. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0197458015002389 10.1016/j.neurobiolaging.2015.04.016 - DOI - PMC - PubMed
-
- Lozano AM, Fosdick L, Chakravarty MM, Leoutsakos J-M, Munro C, Oh E, et al. A Phase II Study of Fornix Deep Brain Stimulation in Mild Alzheimer’s Disease. J Alzheimer’s Dis [Internet]. 2016;54(2):777–87. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27567810%5Cnhttp://www.pubmedcentral.... - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

