Efficacy and safety of direct oral anticoagulants approved for cardiovascular indications: Systematic review and meta-analysis
- PMID: 29795629
- PMCID: PMC5967718
- DOI: 10.1371/journal.pone.0197583
Efficacy and safety of direct oral anticoagulants approved for cardiovascular indications: Systematic review and meta-analysis
Abstract
Background: Direct oral anticoagulants (DOACs) have emerged as promising alternatives to vitamin K antagonists (VKAs) for patients with non-valvular atrial fibrillation (NVAF) or venous thromboembolism (VTE). Few meta-analyses have included all DOACs that have received FDA approval for these cardiovascular indications, and their overall comparisons against VKAs have shortcomings in data and methods. We provide an updated overall assessment of the efficacy and safety of those DOACs at dosages currently approved for NVAF or VTE, in comparison with VKAs.
Methods: We used data from Phase 3 randomized trials that compared an FDA-approved DOAC with VKA for primary prevention of stroke in patients with NVAF or for treatment of acute VTE.
Results: Among trial participants with NVAF, DOAC recipients had a lower risk of stroke or systemic embolism [Pooled Odds Ratio (OR) 0.76, 95% Confidence Interval (CI) (0.68-0.84)], any stroke (0.80, 0.73-0.88), systemic embolism (0.56, 0.34-0.93), and total mortality (0.89, 0.84-0.95). Safety outcomes also showed a lower risk of fatal, major, and intracranial bleeding but higher risk for gastrointestinal bleeding (GIB). Patients with acute VTE randomized to DOACs had comparable risk of recurrent VTE and death (OR 0.88, 95% CI 0.75-1.03), recurrent DVT (0.83, 0.66-1.05), recurrent non-fatal PE (0.97, 0.75-1.25), and total mortality (0.94, 0.79-1.12). Safety outcomes for DOACs showed a lower risk of major, fatal, and intracranial bleeding, but similar risk of GIB.
Conclusions: Patients receiving DOACs for NVAF had predominantly superior efficacy and safety. Patients who were treated with DOACs for acute VTE had non-inferior efficacy, but an overall superior safety profile.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
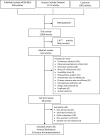




References
-
- Commision E. Pradaxa: Product information 2008 [cited 2017 February 17]. Available from: http://ec.europa.eu/health/documents/community-register/html/h442.htm.
-
- Canada H. Register of Innovative Drugs 2008 [cited 2017 February 17]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/dr....
-
- FDA approves Pradaxa to prevent stroke in people with atrial fibrillation. News & Events: U.S. Food and Drug Administration, 2010.
-
- Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5: 615–621. doi: 10.1161/CIRCOUTCOMES.112.967299 - DOI - PMC - PubMed
-
- Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National Trends in Ambulatory Oral Anticoagulant Use. Am J Med. 2015;128: 1300–1305 e2. doi: 10.1016/j.amjmed.2015.05.044 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

