Propofol elicits autophagy via endoplasmic reticulum stress and calcium exchange in C2C12 myoblast cell line
- PMID: 29795639
- PMCID: PMC5967754
- DOI: 10.1371/journal.pone.0197934
Propofol elicits autophagy via endoplasmic reticulum stress and calcium exchange in C2C12 myoblast cell line
Abstract
In this study, we investigated the relationship between propofol and autophagy and examined whether this relationship depends on ER stress, production of ROS (reactive oxygen species), and disruption of calcium (Ca2+) homeostasis. To this end, we measured C2C12 cell apoptosis in vitro, along with Ca2+ levels; ROS production; and expression of proteins and genes associated with autophagy, Ca2+ homeostasis, and ER stress, including LC3 (microtubule-associate protein 1 light chain 3), p62, AMPK (adenosine 5'-monophosphate (AMP)-activated protein kinase), phosphorylated AMPK, mTOR (the mammalian target of rapamycin), phosphorylated mTOR, CHOP (C/BEP homologous protein), and Grp78/Bip (78 kDa glucose-regulated protein). We found that propofol treatment induced autophagy, ER stress, and Ca2+ release. The ratio of phosphorylated AMPK to AMPK increased, whereas the ratio of phosphorylated mTOR to mTOR decreased. Collectively, the data suggested that propofol induced autophagy in vitro through ER stress, resulting in elevated ROS and Ca2+. Additionally, co-administration of an ER stress inhibitor blunted the effect of propofol.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

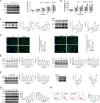


References
-
- Young C, Knudsen N, Hilton A, Reves JG. Sedation in the intensive care unit. Critical care medicine. 2000;28(3):854–66. Epub 2001/02/07. . - PubMed
-
- Wu Q, Zhao Y, Chen X, Zhu M, Miao C. Propofol attenuates BV2 microglia inflammation via NMDA receptor inhibition. Canadian journal of physiology and pharmacology. 2017. Epub 2017/08/18. doi: 10.1139/cjpp-2017-0243 . - DOI - PubMed
-
- Ren G, Zhou Y, Liang G, Yang B, Yang M, King A, et al. General Anesthetics Regulate Autophagy via Modulating the Inositol 1,4,5-Trisphosphate Receptor: Implications for Dual Effects of Cytoprotection and Cytotoxicity. Scientific reports. 2017;7(1):12378 Epub 2017/09/30. doi: 10.1038/s41598-017-11607-0 ; PubMed Central PMCID: PMCPMC5620053. - DOI - PMC - PubMed
-
- Basu S, Mutschler DK, Larsson AO, Kiiski R, Nordgren A, Eriksson MB. Propofol (Diprivan-EDTA) counteracts oxidative injury and deterioration of the arterial oxygen tension during experimental septic shock. Resuscitation. 2001;50(3):341–8. Epub 2001/11/24. . - PubMed
-
- Cui WY, Tian AY, Bai T. Protective effects of propofol on endotoxemia-induced acute kidney injury in rats. Clinical and experimental pharmacology & physiology. 2011;38(11):747–54. Epub 2011/08/10. doi: 10.1111/j.1440-1681.2011.05584.x . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

