Treatment of tardive dyskinesia with VMAT-2 inhibitors: a systematic review and meta-analysis of randomized controlled trials
- PMID: 29795977
- PMCID: PMC5958944
- DOI: 10.2147/DDDT.S133205
Treatment of tardive dyskinesia with VMAT-2 inhibitors: a systematic review and meta-analysis of randomized controlled trials
Abstract
Aim: The aim of this study was to summarize the characteristics, efficacy, and safety of vesicular monoamine transporter-2 (VMAT-2) inhibitors for treating tardive dyskinesia (TD).
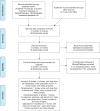
Materials and methods: We conducted a literature search in PubMed, Cochrane Database, and ClinicalTrials.gov, screening for systematic reviews, meta-analyses or double-blind, randomized, placebo-controlled trials (DBRPCTs) reporting efficacy or safety data of VMAT-2 inhibitors (tetrabenazine, deutetrabenazine, and valbenazine) in patients with TD. A random effects meta-analysis of efficacy and safety data from DBRPCTs was performed.
Results: Two acute, 12-week DBRPCTs with deutetrabenazine 12-48 mg/day (n=413) and 4 acute, 4-6-week double-blind trials with valbenazine 12.5-100 mg/day (n=488) were meta-analyzable, without meta-analyzable, high-quality data for tetrabenazine. Regarding reduction in total Abnormal Involuntary Movement Scale (AIMS) scores (primary outcome), both deutetrabenazine (k=2, n=413, standardized mean difference [SMD] =-0.40, 95% confidence interval [CI] =-0.19, -0.62, p<0.001; weighted mean difference (WMD) =-1.44, 95% CI =-0.67, -2.19, p<0.001) and valbenazine (k=4, n=421, SMD =-0.58, 95% CI =-0.26, -0.91, p<0.001; WMD =-2.07, 95% CI =-1.08, -3.05, p<0.001) significantly outperformed placebo. Results were confirmed regarding responder rates (≥50% AIMS total score reduction; deutetrabenazine: risk ratio [RR] =2.13, 95% CI =1.10, 4.12, p=0.024, number-needed-to-treat [NNT] =7, 95% CI =3, 333, p=0.046; valbenazine: RR =3.05, 95% CI =1.81, 5.11, p<0.001, NNT =4, 95% CI =3, 6, p<0.001). Less consistent results emerged from patient-rated global impression-based response (p=0.15) and clinical global impression for deutetrabenazine (p=0.088), and for clinical global impression change for valbenazine (p=0.67). In an open-label extension (OLE) study of deutetrabenazine (≤54 weeks) and a dose-blinded valbenazine study (≤48 weeks), responder rates increased over time. With valbenazine, discontinuation effects were studied, showing TD symptom recurrence towards baseline severity levels within 4 weeks after valbenazine withdrawal. No increased cumulative or specific adverse (AEs) events versus placebo (acute trials) in extension versus acute trial data were observed.
Conclusion: The 2 VMAT-2 inhibitors, valbenazine and deutetrabenazine, are effective in treating TD, both acutely and long-term, without concerns about increased risk of depression or suicide in the TD population. No head-to-head comparison among VMAT-2 inhibitors and no high-quality, meta-analyzable data are available for tetrabenazine in patients with TD.
Keywords: VMAT-2; deutetrabenazine; tardive dyskinesia; tetrabenazine; valbenazine.
Conflict of interest statement
Disclosure Christoph U Correll has been a consultant and/or advisor to or has received honoraria from Alkermes, Allergan, Bristol-Myers Squibb, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, Medavante, Medscape, Neurocrine, Otsuka, Pfizer, ROVI, Sunovion, Takeda, and Teva. He has provided expert testimony for Bristol-Myers Squibb, Janssen, and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck and Pfizer. He received grant support from Janssen and Takeda. He is a shareholder of LB Pharma. John M Kane has been a consultant and/or advisor to or has received honoraria from Alkermes, Allergan, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, Medscape, Neurocrine, Otsuka, Pfizer, Pierre Fabre, Roche, Sunovion, Takeda, and Teva. He has received grant support from Janssen, Lundbeck, and Otsuka. He is a shareholder of The Vanguard Research Group and LB Pharma. The other authors report no other conflicts of interest in this work.
Figures

References
-
- Cummings MA, Proctor GJ, Stahl SM. Deuterium tetrabenazine for tardive dyskinesia. Clin Schizophr Relat Psychoses. 2018;11(4):214–220. - PubMed
-
- Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414–425. - PubMed
-
- Correll CU, Schenk EM. Tardive dyskinesia and new antipsychotics. Curr Opin Psychiatry. 2008;21(2):151–156. - PubMed
-
- Woerner MG, Alvir JM, Saltz BL, Lieberman JA, Kane JM. Prospective study of tardive dyskinesia in the elderly: rates and risk factors. Am J Psychiatry. 1998;155(11):1521–1528. - PubMed
-
- Jeste DV, Caligiuri MP, Paulsen JS, et al. Risk of tardive dyskinesia in older patients. A prospective longitudinal study of 266 outpatients. Arch Gen Psychiatry. 1995;52(9):756–765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

