SB202190 inhibits endothelial cell apoptosis via induction of autophagy and heme oxygenase-1
- PMID: 29796178
- PMCID: PMC5955409
- DOI: 10.18632/oncotarget.25234
SB202190 inhibits endothelial cell apoptosis via induction of autophagy and heme oxygenase-1
Abstract
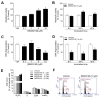
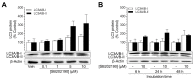
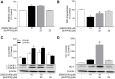
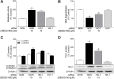
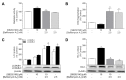
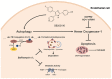
Activation of the p38 mitogen-activated protein kinase (MAPK) pathway has been implicated in various detrimental events finally leading to endothelial dysfunction. The present study therefore investigates the impact of the p38 MAPK inhibitor SB202190 on the expression of the cytoprotective enzyme heme oxygenase-1 (HO-1) as well as metabolic activity, apoptosis and autophagy of endothelial cells. Using human umbilical vein endothelial cells (HUVEC) SB202190 was found to cause a time- and concentration-dependent induction of HO-1 protein. Induction of HO-1 protein expression was mimicked by SB203580, another p38 MAPK inhibitor, but not by SB202474, an inactive structural analogue of p38 MAPK inhibitors. HO-1 induction by both SB202190 and SB203580 was also demonstrated by analysis of mRNA expression. On the functional level, SB202190 was shown to increase metabolic activity and autophagy of HUVEC along with diminishing basal apoptosis. Treatment of cells with tin protoporphyrin IX (SnPPIX), a well-characterised HO-1 enzymatic inhibitor, or HO-1 siRNA left SB202190-modulated metabolic activity and autophagy virtually unaltered but caused a significant reversal of the anti-apoptotic action of SB202190. Conversely, however, HO-1 expression by SB202190 became completely suppressed by the autophagy inhibitor bafilomycin A1. Bafilomycin A1 likewise fully reversed effects of SB202190 on metabolic activity and apoptosis, albeit significantly inducing apoptosis per se. Collectively, this work demonstrates SB202190 to confer upstream induction of autophagy followed by HO-1 induction resulting in potential protective effects against apoptosis. On the other hand, our data oppose HO-1 to contribute to SB202190-mediated increases in metabolic activity and autophagy, respectively.
Keywords: SB202190; apoptosis; autophagy; heme oxygenase-1; p38 MAPK.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Cannabidiol Promotes Endothelial Cell Survival by Heme Oxygenase-1-Mediated Autophagy.Cells. 2020 Jul 16;9(7):1703. doi: 10.3390/cells9071703. Cells. 2020. PMID: 32708634 Free PMC article.
-
Cannabinoid-Induced Autophagy and Heme Oxygenase-1 Determine the Fate of Adipose Tissue-Derived Mesenchymal Stem Cells under Stressful Conditions.Cells. 2020 Oct 15;9(10):2298. doi: 10.3390/cells9102298. Cells. 2020. PMID: 33076330 Free PMC article.
-
Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis.J Exp Med. 2000 Oct 2;192(7):1015-26. doi: 10.1084/jem.192.7.1015. J Exp Med. 2000. PMID: 11015442 Free PMC article.
-
Nitric oxide induces heme oxygenase-1 via mitogen-activated protein kinases ERK and p38.Cell Mol Biol (Noisy-le-grand). 2000 May;46(3):609-17. Cell Mol Biol (Noisy-le-grand). 2000. PMID: 10872747
-
Heme oxygenase-1 enhances autophagy in podocytes as a protective mechanism against high glucose-induced apoptosis.Exp Cell Res. 2015 Oct 1;337(2):146-59. doi: 10.1016/j.yexcr.2015.04.005. Epub 2015 Apr 13. Exp Cell Res. 2015. PMID: 25882498 Review.
Cited by
-
The Progress and Clinical Application of Breast Cancer Organoids.Int J Stem Cells. 2020 Nov 30;13(3):295-304. doi: 10.15283/ijsc20082. Int J Stem Cells. 2020. PMID: 32840232 Free PMC article. Review.
-
Heme Oxgenase-1, a Cardinal Modulator of Regulated Cell Death and Inflammation.Cells. 2021 Feb 28;10(3):515. doi: 10.3390/cells10030515. Cells. 2021. PMID: 33671004 Free PMC article. Review.
-
Cell death-inducing cytotoxicity in truncated KCNQ4 variants associated with DFNA2 hearing loss.Dis Model Mech. 2021 Nov 1;14(11):dmm049015. doi: 10.1242/dmm.049015. Epub 2021 Nov 26. Dis Model Mech. 2021. PMID: 34622280 Free PMC article.
-
Methionine deficiency and its hydroxy analogue influence chicken intestinal 3-dimensional organoid development.Anim Nutr. 2022 Mar;8(1):38-51. doi: 10.1016/j.aninu.2021.06.001. Epub 2021 Sep 3. Anim Nutr. 2022. PMID: 34977374 Free PMC article.
References
-
- Dimmeler S, Zeiher AM. Endothelial cell apoptosis in angiogenesis and vessel regression. Circ Res. 2000;87:434–439. - PubMed
-
- Rössig L, Dimmeler S, Zeiher AM. Apoptosis in the vascular wall and atherosclerosis. Basic Res Cardiol. 2001;96:11–22. - PubMed
-
- Li J, Xiong J, Yang B, Zhou Q, Wu Y, Luo H, Zhou H, Liu N, Li Y, Song Z, Zheng Q. Endothelial cell apoptosis induces TGF-β signaling-dependent host endothelial-mesenchymal transition to promote transplant arteriosclerosis. Am J Transplant. 2015;15:3095–3111. - PubMed
-
- Förstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med. 2008;5:338–349. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

