Treatment of ovarian cancer by targeting the tumor stem cell-associated carbohydrate antigen, Sialyl-Thomsen-nouveau
- PMID: 29796189
- PMCID: PMC5955411
- DOI: 10.18632/oncotarget.25289
Treatment of ovarian cancer by targeting the tumor stem cell-associated carbohydrate antigen, Sialyl-Thomsen-nouveau
Abstract
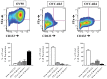
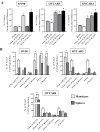
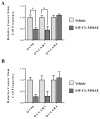
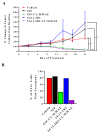
Recurrent ovarian cancer (OvCa) is thought to result in part from the inability to eliminate rare quiescent cancer stem cells (CSCs) that survive cytotoxic chemotherapy and drive tumor resurgence. The Sialyl-Thomsen-nouveau antigen (STn) is a carbohydrate moiety present on protein markers of CSCs in pancreatic, colon, and gastric malignancies. We have demonstrated that human OvCa cell lines contain varying levels of cells that independently express either STn or the ovarian CSC marker CD133. Here we determine co-expression of STn and CD133 in a subset of human OvCa cell lines. Analyses of colony and sphere forming capacity and of response to standard-of-care cytotoxic therapy suggest a subset of OvCa STn+ cells display some CSC features. The effect of the anti-STn antibody-drug conjugates (ADCs) S3F-CL-MMAE and 2G12-2B2-CL-MMAE on OvCa cell viability in vitro and in vivo was also assessed. Treatment with S3F-CL-MMAE reduced the viability of two of three OvCa cell lines in vitro and exposure to either S3F-CL-MMAE or 2G12-2B2-CL-MMAE reduced OVCAR3-derived xenograft volume in vivo, depleting STn+ tumor cells. In summary, STn+ cells demonstrate some stem-like properties and specific therapeutic targeting of STn in ovarian tumors may be an effective clinical strategy to eliminate both STn+ CSC and STn+ non-CSC populations.
Keywords: antibody-drug conjugate; cancer stem cell; ovarian cancer; sialyl-Tn; tumor-associated carbohydrate antigen.
Conflict of interest statement
CONFLICTS OF INTEREST Rosemary Foster, Silvia Fatima Hernandez, Linah Al-Alem, Chiara Bellio, Bianca Zarrella, Whitfield B. Growdon, and Kristen Starbuck have no conflicts of interest. Bo Rueda receives stock options for serving as a member of the scientific advisory committee for Siamab Therapeutics, Inc. David Eavarone, Jillian Prendergast, Jenna Stein, Daniel Dransfield and Jeffery Behrens are all employed by Siamab Therapeutics, Inc. This research may lead to the development of products which may be owned by and/or licensed to Siamab Therapeutics, Inc. in which they have a business and/or financial interest.
Figures



Similar articles
-
161Tb-Based Anti-L1CAM Radioimmunotherapy Shows Superior Efficacy in Eliminating Ovarian Cancer Stem Cells Compared with 177Lu in Preclinical Models of Ovarian Cancer.J Nucl Med. 2025 Jul 1;66(7):1091-1096. doi: 10.2967/jnumed.124.269078. J Nucl Med. 2025. PMID: 40473465
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Taxane monotherapy regimens for the treatment of recurrent epithelial ovarian cancer.Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD008766. doi: 10.1002/14651858.CD008766.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866378 Free PMC article.
Cited by
-
Unmasking the Deceptive Nature of Cancer Stem Cells: The Role of CD133 in Revealing Their Secrets.Int J Mol Sci. 2023 Jun 30;24(13):10910. doi: 10.3390/ijms241310910. Int J Mol Sci. 2023. PMID: 37446085 Free PMC article. Review.
-
Targeting Galectin 3 illuminates its contributions to the pathology of uterine serous carcinoma.Br J Cancer. 2024 May;130(9):1463-1476. doi: 10.1038/s41416-024-02621-x. Epub 2024 Mar 4. Br J Cancer. 2024. PMID: 38438589 Free PMC article.
-
Truncated O-glycans promote epithelial-to-mesenchymal transition and stemness properties of pancreatic cancer cells.J Cell Mol Med. 2019 Oct;23(10):6885-6896. doi: 10.1111/jcmm.14572. Epub 2019 Aug 7. J Cell Mol Med. 2019. PMID: 31389667 Free PMC article.
-
Glycan diversity in ovarian cancer: Unraveling the immune interplay and therapeutic prospects.Semin Immunopathol. 2024 Oct 21;46(6):16. doi: 10.1007/s00281-024-01025-6. Semin Immunopathol. 2024. PMID: 39432076 Free PMC article. Review.
-
From "Serum Sickness" to "Xenosialitis": Past, Present, and Future Significance of the Non-human Sialic Acid Neu5Gc.Front Immunol. 2019 Apr 17;10:807. doi: 10.3389/fimmu.2019.00807. eCollection 2019. Front Immunol. 2019. PMID: 31057542 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. https://doi.org/10.3322/caac.21442. - DOI - PubMed
-
- Hodeib M, Eskander RN, Bristow RE. New paradigms in the surgical and adjuvant treatment of ovarian cancer. Minerva Ginecol. 2014;66:179–92. - PubMed
-
- Curley MD, Garrett LA, Schorge JO, Foster R, Rueda BR. Evidence for cancer stem cells contributing to the pathogenesis of ovarian cancer. Front Biosci (Landmark Ed) 2011;16:368–92. - PubMed
-
- Foster R, Buckanovich RJ, Rueda BR. Ovarian cancer stem cells: working towards the root of stemness. Cancer Lett. 2013;338:147–57. https://doi.org/10.1016/j.canlet.2012.10.023. - DOI - PubMed
-
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. https://doi.org/10.1073/pnas.0530291100. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

