The prevalence of patient engagement in published trials: a systematic review
- PMID: 29796308
- PMCID: PMC5963039
- DOI: 10.1186/s40900-018-0099-x
The prevalence of patient engagement in published trials: a systematic review
Abstract
Plain english summary: With the growing movement to engage patients in research, questions are being asked about who is engaging patients and how they are being engaged. Internationally, research groups are supporting and funding patient-oriented research studies that engage patients in the identification of research priorities and the design, conduct and uptake of research. As we move forward, we need to know what meaningful patient engagement looks like, how it benefits research and clinical practice, and what are the barriers to patient engagement?We conducted a review of the published literature looking for trials that report engaging patients in the research. We included both randomized controlled trials and non-randomized comparative trials. We looked at these trials for important study characteristics, including how patients were engaged, to better understand the practices used in trials. Importantly, we also discuss the number of trials reporting patient engagement practices relative to all published trials. We found that very few trials report any patient engagement activities even though it is widely supported by many major funding organizations. The findings of our work will advance patient-oriented research by showing how patients can be engaged and by stressing that patient engagement practices need to be better reported.
Background: Patient-Oriented Research (POR) is research informed by patients and is centred on what is of importance to them. A fundamental component of POR is that patients are included as an integral part of the research process from conception to dissemination and implementation, and by extension, across the research continuum from basic research to pragmatic trials [J Comp Eff Res 2012, 1:181-94, JAMA 2012, 307:1587-8]. Since POR's inception, questions have been raised as to how best to achieve this goal.We conducted a systematic review of randomized controlled trials and non-randomized comparative trials that report engaging patients in their research. Our main goal was to describe the characteristics of published trials engaging patients in research, and to identify the extent of patient engagement activities reported in these trials.
Methods: The MEDLINE®, EMBASE®, Cinahl, PsycINFO, Cochrane Methodology Registry, and Pubmed were searched from May 2011 to June 16th, 2016. Title, abstract and full text screening of all reports were conducted independently by two reviewers. Data were extracted from included trials by one reviewer and verified by a second. All trials that report patient engagement for the purposes of research were included.
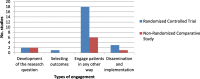
Results: Of the 9490 citations retrieved, 2777 were reviewed at full text, of which 23 trials were included. Out of the 23 trials, 17 were randomized control trials, and six were non-randomized comparative trials. The majority of these trials (83%, 19/23) originated in the United States and United Kingdom. The trials engaged a range of 2-24 patients/ community representatives per study. Engagement of children and minorities occurred in 13% (3/23) and 26% (6/23) of trials; respectively. Engagement was identified in the development of the research question, the selection of study outcomes, and the dissemination and implementation of results.
Conclusions: The prevalence of patient engagement in patient-oriented interventional research is very poor with 23 trials reporting activities engaging patients. Research dedicated to determining the best practice for meaningful engagement is still needed, but adequate reporting measures also need to be defined.
Keywords: Clinical trials; Patient engagement; Patient-oriented research; Systematic review.
Conflict of interest statement
Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Patient-Centered Outcomes Research Institute The PCORI Methodology Report. [Internet]. https://www.pcori.org/research-results/about-our-research/research-metho.... Accessed 5 Jan 2018.
-
- Canada Institutes for Health Research Strategy for Patient-Oriented Research. The Challenge: Health Research and Health Care. [Internet]. http://www.cihr-irsc.gc.ca/e/47473.html. Accessed 6 July 2016.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous