Decreasing cytosolic translation is beneficial to yeast and human Tafazzin-deficient cells
- PMID: 29796387
- PMCID: PMC5961916
- DOI: 10.15698/mic2018.05.629
Decreasing cytosolic translation is beneficial to yeast and human Tafazzin-deficient cells
Abstract
Cardiolipin (CL) optimizes diverse mitochondrial processes, including oxidative phosphorylation (OXPHOS). To function properly, CL needs to be unsaturated, which requires the acyltransferase Tafazzin (TAZ). Loss-of-function mutations in the TAZ gene are responsible for the Barth syndrome (BTHS), a rare X-linked cardiomyopathy, presumably because of a diminished OXPHOS capacity. Herein we show that a partial inhibition of cytosolic protein synthesis, either chemically with the use of cycloheximide or by specific genetic mutations, fully restores biogenesis and the activity of the oxidative phosphorylation system in a yeast BTHS model (taz1Δ). Interestingly, the defaults in CL were not suppressed, indicating that they are not primarily responsible for the OXPHOS deficiency in taz1Δ yeast. Low concentrations of cycloheximide in the picomolar range were beneficial to TAZ-deficient HeLa cells, as evidenced by the recovery of a good proliferative capacity. These findings reveal that a diminished capacity of CL remodeling deficient cells to preserve protein homeostasis is likely an important factor contributing to the pathogenesis of BTHS. This in turn, identifies cytosolic translation as a potential therapeutic target for the treatment of this disease.
Keywords: Barth syndrome; cycloheximide; cardiolipin remodeling; cytosolic protein synthesis; mitochondrial disease; oxidative phosphorylation.
Conflict of interest statement
Conflict of interest: The authors declare no competing or financial interests.
Figures
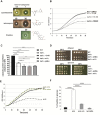
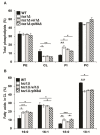
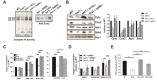

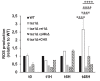
References
-
- Barth PG, Scholte HR, Berden JA, Van der Klei-Van Moorsel JM, Luyt-Houwen IE, Van 't Veer-Korthof ET, Van der Harten JJ, Sobotka-Plojhar MA. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J Neurol Sci. 1983;62(1-3):327–355. doi: 10.1016/0022-510X(83)90209-5. - DOI - PubMed
-
- Schlame M, Haldar D. Cardiolipin is synthesized on the matrix side of the inner membrane in rat liver mitochondria. J Biol Chem. 1993;268(1):74–79. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
