Emerging Applications of Optical Coherence Tomography Angiography (OCTA) in neurological research
- PMID: 29796403
- PMCID: PMC5956832
- DOI: 10.1186/s40662-018-0104-3
Emerging Applications of Optical Coherence Tomography Angiography (OCTA) in neurological research
Abstract
Purpose: To review the clinical and research value of optical coherence tomography angiography (OCTA) in the field of neurology.
Methods: Current literature involving OCTA were reviewed through PubMed using the search terms "optical coherence tomography angiography", with "multiple sclerosis", "Alzheimer's disease", "optic neuropathy", or other closely-related terms.
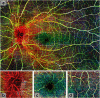
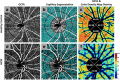
Results: OCTA has been applied in research to advance our understanding of the pathobiology of neurological disorders. OCTA-derived blood flow and vessel density measures are altered in multiple sclerosis (MS), Alzheimer's disease (AD), and various optic neuropathies (ON) in varying regions of the posterior segment vasculature of the eye. These emerging research findings support the occurrence of retinal vascular alterations across a host of neurological disorders and raise the possibility that vasculopathy can be clinically relevant since it contributes to the pathobiology of several neurological disorders.
Conclusion: OCTA may be beneficial for neurological research. Additional investigations using OCTA in neurological disorders will help to further validate its clinical and research utilities in terms of characterizing the role of vasculopathy in neurological disorders.
Keywords: Alzheimer’s disease; Multiple sclerosis; Neurology; Optic neuropathy; Optical coherence tomography angiography.
Conflict of interest statement
Not applicable.The authors declare that they have no competing interests.
Figures



References
-
- Carpineto P, Mastropasqua R, Marchini G, Toto L, Di Nicola M, Di Antonio L. Reproducibility and repeatability of foveal avascular zone measurements in healthy subjects by optical coherence tomography angiography. Br J Ophthalmol. 2016;100(5):671–676. doi: 10.1136/bjophthalmol-2015-307330. - DOI - PubMed
-
- Huang Y, Zhang Q, Thorell MR, An L, Durbin MK, Laron M, et al. Swept-source OCT angiography of the retinal vasculature using intensity differentiation-based optical microangiography algorithms. Ophthalmic Surg Lasers Imaging Retina. 2014;45(5):382–389. doi: 10.3928/23258160-20140909-08. - DOI - PMC - PubMed
-
- Nehemy MB, Brocchi DN, Veloso CE. Optical Coherence Tomography Angiography Imaging of Quiescent Choroidal Neovascularization in Age-Related Macular Degeneration. Ophthalmic Surg Lasers Imaging Retina. 2015;46(10):1056–7. - PubMed
-
- de Carlo TE, Chin AT, Joseph T, Baumal CR, Witkin AJ, Duker JS, et al. Distinguishing Diabetic Macular Edema From Capillary Nonperfusion Using Optical Coherence Tomography Angiography. Ophthalmic Surg Lasers Imaging Retina. 2016;47(2):108–14. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

