Oncolytic virotherapy in glioblastoma patients induces a tumor macrophage phenotypic shift leading to an altered glioblastoma microenvironment
- PMID: 29796615
- PMCID: PMC6176807
- DOI: 10.1093/neuonc/noy082
Oncolytic virotherapy in glioblastoma patients induces a tumor macrophage phenotypic shift leading to an altered glioblastoma microenvironment
Abstract
Background: Immunosuppressive protumoral M2 macrophages are important in pathogenesis, progression, and therapy resistance in glioblastoma (GBM) and provide a target for therapy. Recently oncolytic virotherapy in murine models was shown to change these M2 macrophages toward the pro-inflammatory and antitumoral M1 phenotype. Here we study the effects of the oncolytic virotherapy Delta24-RGD in humans, using both in vitro models and patient material.
Methods: Human monocyte-derived macrophages were co-cultured with Delta24-RGD-infected primary glioma stem-like cells (GSCs) and were analyzed for their immunophenotype, cytokine expression, and secretion profiles. Cerebrospinal fluid (CSF) from 18 Delta24-RGD-treated patients was analyzed for inflammatory cytokine levels, and the effects of these CSF samples on macrophage phenotype in vitro were determined. In addition, tumor macrophages in resected material from a Delta24-RGD-treated GBM patient were compared with 5 control GBM patient samples by flow cytometry.
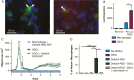
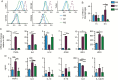
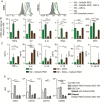
Results: Human monocyte-derived M2 macrophages co-cultured with Delta24-RGD-infected GSCs shifted toward an M1-immunophenotype, coinciding with pro-inflammatory gene expression and cytokine production. This phenotypic switch was induced by the concerted effects of a change in tumor-produced soluble factors and the presence of viral particles. CSF samples from Delta24-RGD-treated GBM patients revealed cytokine levels indicative of a pro-inflammatory microenvironment. Furthermore, tumoral macrophages in a Delta24-RGD-treated patient showed significantly greater M1 characteristics than in control GBM tissue.
Conclusion: Together these in vitro and patient studies demonstrate that local Delta24-RGD therapy may provide a therapeutic tool to promote a prolonged shift in the protumoral M2 macrophages toward M1 in human GBM, inducing a pro-inflammatory and potentially tumor-detrimental microenvironment.
Figures


References
-
- Movahedi K, Laoui D, Gysemans C, et al. . Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728–5739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

