Premature Expression of FOXO1 in Developing Mouse Pituitary Results in Anterior Lobe Hypoplasia
- PMID: 29796621
- PMCID: PMC6456930
- DOI: 10.1210/en.2018-00107
Premature Expression of FOXO1 in Developing Mouse Pituitary Results in Anterior Lobe Hypoplasia
Abstract
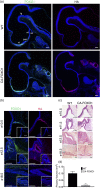
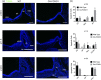
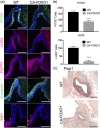
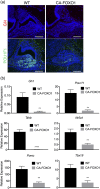
The process by which the somatotrope lineage emerges in the developing pituitary is regulated by the activity of specific signaling and transcription factors expressed during development. We set out to understand the contribution of FOXO1 to that process by using a mouse model in which FOXO1 is prematurely expressed in the pituitary primordium. Expression of FOXO1 in the oral ectoderm as early as embryonic day (e)9.5 resulted in pituitary gland hypoplasia and reduced expression of anterior lobe hormone transcripts at e18.5. Of note, the relative numbers of somatotropes and thyrotropes were also decreased at e18.5. LHX3 and PITX2, markers of pituitary identity, were present in a reduced number of cells during the formation of the Rathke pouch. Thus, premature expression of FOXO1 may affect adoption of pituitary identity during differentiation. Our results demonstrate that the timing of FOXO1 activation affects its role in pituitary gland organogenesis and somatotrope differentiation.
Figures



Similar articles
-
Foxo1 Is Required for Normal Somatotrope Differentiation.Endocrinology. 2016 Nov;157(11):4351-4363. doi: 10.1210/en.2016-1372. Epub 2016 Sep 15. Endocrinology. 2016. PMID: 27631552 Free PMC article.
-
Reduced expression of the LIM-homeobox gene Lhx3 impairs growth and differentiation of Rathke's pouch and increases cell apoptosis during mouse pituitary development.Mech Dev. 2006 Aug;123(8):605-13. doi: 10.1016/j.mod.2006.06.005. Epub 2006 Jun 14. Mech Dev. 2006. PMID: 16859901
-
Specification of pituitary cell lineages by the LIM homeobox gene Lhx3.Science. 1996 May 17;272(5264):1004-7. doi: 10.1126/science.272.5264.1004. Science. 1996. PMID: 8638120
-
Pituitary development: regulatory codes in mammalian organogenesis.Science. 2002 Mar 22;295(5563):2231-5. doi: 10.1126/science.1062736. Science. 2002. PMID: 11910101 Review.
-
Molecular Mechanisms Governing Embryonic Differentiation of Pituitary Somatotropes.Trends Endocrinol Metab. 2018 Jul;29(7):510-523. doi: 10.1016/j.tem.2018.04.009. Epub 2018 May 11. Trends Endocrinol Metab. 2018. PMID: 29759686 Review.
Cited by
-
FOXO Transcription Factors Are Required for Normal Somatotrope Function and Growth.Endocrinology. 2022 Feb 1;163(2):bqab263. doi: 10.1210/endocr/bqab263. Endocrinology. 2022. PMID: 34971379 Free PMC article.
-
The interplay between FOXO1 and glucocorticoid signaling in promoting the terminal differentiation of somatotropes.Mol Cell Endocrinol. 2025 Sep 1;606:112573. doi: 10.1016/j.mce.2025.112573. Epub 2025 May 15. Mol Cell Endocrinol. 2025. PMID: 40381980
-
Overview of chromatin regulatory processes during surface ectodermal development and homeostasis.Dev Biol. 2024 Nov;515:30-45. doi: 10.1016/j.ydbio.2024.07.001. Epub 2024 Jul 4. Dev Biol. 2024. PMID: 38971398 Review.
References
-
- Savage JJ, Yaden BC, Kiratipranon P, Rhodes SJ. Transcriptional control during mammalian anterior pituitary development. Gene. 2003;319:1–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

