Identification of serum glycoprotein ligands for the immunomodulatory receptor blood dendritic cell antigen 2
- PMID: 29796630
- PMCID: PMC6054153
- DOI: 10.1093/glycob/cwy050
Identification of serum glycoprotein ligands for the immunomodulatory receptor blood dendritic cell antigen 2
Abstract
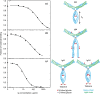
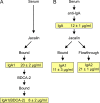
Blood dendritic cell antigen 2 (BDCA-2) is a C-type lectin found on the surface of plasmacytoid dendritic cells. It functions as a glycan-binding receptor that downregulates the production of type I interferons and thus plays a role in oligosaccharide-mediated immunomodulation. The carbohydrate recognition domain in BDCA-2 binds selectively to galactose-terminated bi-antennary glycans. Because the plasmacytoid dendritic cells function in a plasma environment rich in glycoproteins, experiments have been undertaken to identify endogenous ligands for blood dendritic cell antigen 2. A combination of blotting, affinity chromatography and proteomic analysis reveals that serum glycoprotein ligands for BDCA-2 include IgG, IgA and IgM. Compared to binding of IgG, which was previously described, IgA and IgM bind with higher affinity. The association constants for the different subclasses of immunoglobulins are below and roughly proportional to the serum concentrations of these glycoprotein ligands. Binding to the other main serum glycoprotein ligand, α2-macroglobulin, is independent of whether this protease inhibitor is activated. Binding to all of these glycoprotein ligands is mediated predominantly by bi-antennary glycans in which each branch bears a terminal galactose residue. The different affinities of the glycoprotein ligands reflect the different numbers of these galactose-terminated glycans and their degree of exposure on the native glycoproteins. The results suggest that normal serum levels of immunoglobulins could downmodulate interferon stimulation of further antibody production.
Figures




Similar articles
-
Interactions that define the arrangement of sugar-binding sites in BDCA-2 and dectin-2 dimers.Glycobiology. 2024 Dec 10;34(12):cwae082. doi: 10.1093/glycob/cwae082. Glycobiology. 2024. PMID: 39361900 Free PMC article.
-
A Novel Mechanism for Binding of Galactose-terminated Glycans by the C-type Carbohydrate Recognition Domain in Blood Dendritic Cell Antigen 2.J Biol Chem. 2015 Jul 3;290(27):16759-71. doi: 10.1074/jbc.M115.660613. Epub 2015 May 20. J Biol Chem. 2015. PMID: 25995448 Free PMC article.
-
HCV glycoprotein E2 is a novel BDCA-2 ligand and acts as an inhibitor of IFN production by plasmacytoid dendritic cells.Blood. 2012 Nov 29;120(23):4544-51. doi: 10.1182/blood-2012-02-413286. Epub 2012 Oct 10. Blood. 2012. PMID: 23053572
-
Signaling and immune regulatory role of the dendritic cell immunoreceptor (DCIR) family lectins: DCIR, DCAR, dectin-2 and BDCA-2.Immunobiology. 2004;209(1-2):179-90. doi: 10.1016/j.imbio.2004.03.004. Immunobiology. 2004. PMID: 15481152 Review.
-
Plasmacytoid dendritic cells: from specific surface markers to specific cellular functions.Hum Immunol. 2002 Dec;63(12):1133-48. doi: 10.1016/s0198-8859(02)00752-8. Hum Immunol. 2002. PMID: 12480257 Review.
Cited by
-
Inhibitory receptors of plasmacytoid dendritic cells as possible targets for checkpoint blockade in cancer.Front Immunol. 2024 Mar 5;15:1360291. doi: 10.3389/fimmu.2024.1360291. eCollection 2024. Front Immunol. 2024. PMID: 38504978 Free PMC article. Review.
-
cGAS-STING pathway mediates activation of dendritic cell sensing of immunogenic tumors.Cell Mol Life Sci. 2024 Mar 21;81(1):149. doi: 10.1007/s00018-024-05191-6. Cell Mol Life Sci. 2024. PMID: 38512518 Free PMC article. Review.
-
Interactions that define the arrangement of sugar-binding sites in BDCA-2 and dectin-2 dimers.Glycobiology. 2024 Dec 10;34(12):cwae082. doi: 10.1093/glycob/cwae082. Glycobiology. 2024. PMID: 39361900 Free PMC article.
-
A type I interferon regulatory network for human plasmacytoid dendritic cells based on heparin, membrane-bound and soluble BDCA-2.Proc Natl Acad Sci U S A. 2024 Mar 19;121(12):e2312404121. doi: 10.1073/pnas.2312404121. Epub 2024 Mar 13. Proc Natl Acad Sci U S A. 2024. PMID: 38478694 Free PMC article.
-
The Dectin-1 and Dectin-2 clusters: C-type lectin receptors with fundamental roles in immunity.EMBO Rep. 2024 Dec;25(12):5239-5264. doi: 10.1038/s44319-024-00296-2. Epub 2024 Oct 31. EMBO Rep. 2024. PMID: 39482490 Free PMC article. Review.
References
-
- Arnold JN, Wallis R, Willis AC, Harvey DJ, Royle L, Dwek RA, Rudd PM, Sim RB. 2006. Interaction of mannan binding lectin with α2 macroglobulin via exposed oligomannose glycans: a conserved feature of the thiol ester protein family? J Biol Chem. 281:6955–6963. - PubMed
-
- Borth W. 1992. α2-Macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 6:3345–3353. - PubMed
-
- de Beer FC, Pepys MB. 1982. Isolation of human C-reactive protein and serum amyloid P component. J Immunol Meth. 50:17–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

