Stability of local secondary structure determines selectivity of viral RNA chaperones
- PMID: 29796667
- PMCID: PMC6125681
- DOI: 10.1093/nar/gky394
Stability of local secondary structure determines selectivity of viral RNA chaperones
Abstract
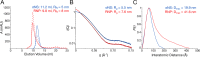
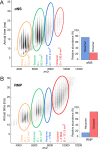
To maintain genome integrity, segmented double-stranded RNA viruses of the Reoviridae family must accurately select and package a complete set of up to a dozen distinct genomic RNAs. It is thought that the high fidelity segmented genome assembly involves multiple sequence-specific RNA-RNA interactions between single-stranded RNA segment precursors. These are mediated by virus-encoded non-structural proteins with RNA chaperone-like activities, such as rotavirus (RV) NSP2 and avian reovirus σNS. Here, we compared the abilities of NSP2 and σNS to mediate sequence-specific interactions between RV genomic segment precursors. Despite their similar activities, NSP2 successfully promotes inter-segment association, while σNS fails to do so. To understand the mechanisms underlying such selectivity in promoting inter-molecular duplex formation, we compared RNA-binding and helix-unwinding activities of both proteins. We demonstrate that octameric NSP2 binds structured RNAs with high affinity, resulting in efficient intramolecular RNA helix disruption. Hexameric σNS oligomerizes into an octamer that binds two RNAs, yet it exhibits only limited RNA-unwinding activity compared to NSP2. Thus, the formation of intersegment RNA-RNA interactions is governed by both helix-unwinding capacity of the chaperones and stability of RNA structure. We propose that this protein-mediated RNA selection mechanism may underpin the high fidelity assembly of multi-segmented RNA genomes in Reoviridae.
Figures







Similar articles
-
Evidence that avian reovirus σNS is an RNA chaperone: implications for genome segment assortment.Nucleic Acids Res. 2015 Aug 18;43(14):7044-57. doi: 10.1093/nar/gkv639. Epub 2015 Jun 24. Nucleic Acids Res. 2015. PMID: 26109354 Free PMC article.
-
Reovirus Nonstructural Protein σNS Acts as an RNA Stability Factor Promoting Viral Genome Replication.J Virol. 2018 Jul 17;92(15):e00563-18. doi: 10.1128/JVI.00563-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29769334 Free PMC article.
-
Structure-function analysis of rotavirus NSP2 octamer by using a novel complementation system.J Virol. 2006 Aug;80(16):7984-94. doi: 10.1128/JVI.00172-06. J Virol. 2006. PMID: 16873255 Free PMC article.
-
Nonstructural proteins involved in genome packaging and replication of rotaviruses and other members of the Reoviridae.Virus Res. 2004 Apr;101(1):57-66. doi: 10.1016/j.virusres.2003.12.006. Virus Res. 2004. PMID: 15010217 Review.
-
Rotavirus NSP2: A Master Orchestrator of Early Viral Particle Assembly.Viruses. 2024 May 21;16(6):814. doi: 10.3390/v16060814. Viruses. 2024. PMID: 38932107 Free PMC article. Review.
Cited by
-
Uncovering protein conformational dynamics within two-component viral biomolecular condensates.Protein Sci. 2025 Jul;34(7):e70181. doi: 10.1002/pro.70181. Protein Sci. 2025. PMID: 40521608 Free PMC article.
-
Structural rearrangements allow nucleic acid discrimination by type I-D Cascade.Nat Commun. 2022 May 20;13(1):2829. doi: 10.1038/s41467-022-30402-8. Nat Commun. 2022. PMID: 35595728 Free PMC article.
-
Quantitative Characterization of Three Carbonic Anhydrase Inhibitors by LESA Mass Spectrometry.J Am Soc Mass Spectrom. 2022 Jul 6;33(7):1168-1175. doi: 10.1021/jasms.2c00024. Epub 2022 Jun 8. J Am Soc Mass Spectrom. 2022. PMID: 35675480 Free PMC article.
-
Avian Reovirus: From Molecular Biology to Pathogenesis and Control.Viruses. 2024 Dec 23;16(12):1966. doi: 10.3390/v16121966. Viruses. 2024. PMID: 39772272 Free PMC article. Review.
-
Genome packaging in multi-segmented dsRNA viruses: distinct mechanisms with similar outcomes.Curr Opin Virol. 2018 Dec;33:106-112. doi: 10.1016/j.coviro.2018.08.001. Epub 2018 Aug 23. Curr Opin Virol. 2018. PMID: 30145433 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

