Development and Evaluation of a Reverse-Entry Ion Source Orbitrap Mass Spectrometer
- PMID: 29796735
- PMCID: PMC6251776
- DOI: 10.1007/s13361-018-1976-0
Development and Evaluation of a Reverse-Entry Ion Source Orbitrap Mass Spectrometer
Erratum in
-
Correction to: JASMS, Volume 30, Number 1, January 2019.J Am Soc Mass Spectrom. 2019 Mar 1;30(3):561. J Am Soc Mass Spectrom. 2019. PMID: 31922744
Abstract
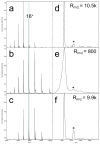
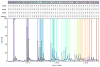
As a step towards development of a high-resolution ion mobility mass spectrometer using the orbitrap mass analyzer platform, we describe herein a novel reverse-entry ion source (REIS) coupled to the higher-energy C-trap dissociation (HCD) cell of an orbitrap mass spectrometer with extended mass range. Development of the REIS is a first step in the development of a drift tube ion mobility-orbitrap MS. The REIS approach retains the functionality of the commercial instrument ion source which permits the uninterrupted use of the instrument during development as well as performance comparisons between the two ion sources. Ubiquitin (8.5 kDa) and lipid binding to the ammonia transport channel (AmtB, 126 kDa) protein complex were used as model soluble and membrane proteins, respectively, to evaluate the performance of the REIS instrument. Mass resolution obtained with the REIS is comparable to that obtained using the commercial ion source. The charge state distributions for ubiquitin and AmtB obtained on the REIS are in agreement with previous studies which suggests that the REIS-orbitrap EMR retains native structure in the gas phase. Graphical Abstract ᅟ.
Keywords: Exactive; Instrument development; Ion source; Membrane protein; Orbitrap.
Figures


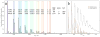
References
-
- Lanucara F, Holman SW, Gray CJ, Eyers CE. The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics. Nat Chem. 2014;6:281–294. - PubMed
-
- von Helden G, Wyttenbach T, Bowers MT. Inclusion of a MALDI ion source in the ion chromatography technique: conformational information on polymer and biomolecular ions. Int J Mass Spectrom Ion Process. 1995;146–147:349–364.
-
- Wittmer D, Chen YH, Luckenbill BK, Hill HH. Electrospray Ionization Ion Mobility Spectrometry. Anal Chem. 1994;66:2348–2355.
-
- Jurneczko E, Barran PE. How useful is ion mobility mass spectrometry for structural biology? The relationship between protein crystal structures and their collision cross sections in the gas phase. Analyst. 2011;136:20–28. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

