Factors associated with acute medication overuse in people with migraine: results from the 2017 migraine in America symptoms and treatment (MAST) study
- PMID: 29797100
- PMCID: PMC5968010
- DOI: 10.1186/s10194-018-0865-z
Factors associated with acute medication overuse in people with migraine: results from the 2017 migraine in America symptoms and treatment (MAST) study
Abstract
Background: The MAST Study is a longitudinal, cross-sectional survey study of US adults with migraine. These analyses were conducted to estimate rates of acute medication overuse (AMO) and determine associations of AMO with individual and headache characteristics.
Methods: Eligible respondents had ICHD-3-beta migraine, reported ≥3 monthly headache days (MHDs) in the past 3 months, ≥1 MHD in the past 30 days, and currently took acute headache medication. AMO was defined according to ICHD-3-beta thresholds for monthly days of medication taking when diagnosing medication overuse headache.
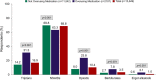
Results: Eligible respondents (N = 13,649) had a mean age of 43.4 ± 13.6 years; most were female (72.9%) and Caucasian (81.9%). Altogether, 15.4% of respondents met criteria for AMO. Compared with those not overusing medications, respondents with AMO were significantly more likely to be taking triptans (31.3% vs 14.2%), opioids (23.8% vs 8.0%), barbiturates (7.8% vs 2.7%), and ergot alkaloids (3.1% vs 0.6%) and significantly less likely to be taking NSAIDs (63.3% vs 69.8%) (p < 0.001 for all comparisons). Respondents with AMO had significantly more MHDs (12.9 ± 8.6 vs 4.3 ± 4.3, p < 0.001); higher migraine symptom severity (17.8 ± 2.7 vs 16.4 ± 3.0, p < 0.001), higher pain intensity scores (7.4 vs 6.5, p < 0.001); and higher rates of cutaneous allodynia (53.7% vs 37.5%, p < 0.001). Adjusted for MHDs, the odds of AMO were increased by each additional year of age (OR 1.02, 95% CI 1.02, 1.03); being married (OR 1.19, 95% CI 1.06, 1.34); smoking (OR 1.54, 95% CI 1.31, 1.81); having psychological symptoms (OR 1.62, 95% CI 1.43, 1.83) or cutaneous allodynia (OR 1.22, 95% CI 1.08, 1.37); and greater migraine symptom severity (OR 1.06, 95% CI 1.04, 1.09) and pain intensity (OR 1.27, 95% CI 1.22, 1.32). Cutaneous allodynia increased the risk of AMO by 61% in males (OR 1.61, 95% CI 1.28, 2.03) but did not increase risk in females (OR 1.08, 95% CI 0.94, 1.25).
Conclusions: AMO was present in 15% of respondents with migraine. AMO was associated with higher symptom severity scores, pain intensity, and rates of cutaneous allodynia. AMO was more likely in triptan, opioid, and barbiturate users but less likely in NSAID users. Cutaneous allodynia was associated with AMO in men but not women. This gender difference merits additional exploration.
Keywords: Adults; Allodynia; Epidemiology; Medication overuse headache; Migraine.
Conflict of interest statement
Ethics approval and consent to participate
The information and consent form, as well as the MAST survey instrument, were reviewed by Ethical and Independent Review Services (Independence, MO), which granted an exemption under (45 CFR 46.101 [2]) and certified the exemption status of the MAST Study (#16106–01) on 31 August 2016. Before initiating the survey, respondents read a description of the study, confirmed that they understood the purpose and conduct of the study, and electronically signed informed consent to participate.
Competing interests
Todd Schwedt owns stock options from Nocira and Second Opinion and receives royalties from UpToDate. He receives grant support from the National Institutes of Health, the US Department of Defense, the Patient Centered Outcomes Research Institute, the American Migraine Foundation, Arizona State University, and the Mayo Clinic. He serves as a consultant, advisory board member, or has received honoraria from Alder, Allergan, Amgen, American Headache Society, Autonomic Technologies, Avanir, Dr. Reddy’s Laboratories/Promius Pharma, Eli Lilly and Company, Ipsen Biosciences, Nocira, Novartis, and Teva.
Aftab Alam is an employee of Dr. Reddy’s Laboratories and owns stock in the company.
Michael Reed receives support from Allergan, Dr. Reddy’s Laboratories/Promius Pharma, Eli Lilly and Conpamy, GlaxoSmithKline, Merck & Co., Inc., NuPathe, Novartis, and Ortho-McNeil, via grants to the National Headache Foundation.
Kristina Fanning receives support from Allergan, Dr. Reddy’s Laboratories/Promius Pharma, Eli Lilly and Company, GlaxoSmithKline, Merck & Co., Inc., NuPathe, Novartis, and Ortho-McNeil, via grants to the National Headache Foundation.
Sagar Munjal is an employee of Dr. Reddy’s Laboratories and owns stock in the company.
Dawn Buse receives grant support and honoraria from Allergan, Avanir, Dr. Reddy’s Laboratories/Promius Pharma, and Eli Lilly and Company. She is an employee of Montefiore Medical Center, which has received research support funded by Allergan, CoLucid, Endo Pharmaceuticals, GlaxoSmithKline, MAP Pharmaceuticals, Merck, NuPathe, Novartis, Ortho-McNeil, and Zogenix, via grants to the National Headache Foundation. She serves on the editorial boards of the Current Pain and Headache Reports, Journal of Headache and Pain, Pain Medicine News, and Pain Pathways magazine.
Dr. Dodick has received compensation from serving on advisory boards and/or consulting within the past 5 years for: Allergan, Amgen, Novartis, Alder, Arteaus, Pfizer, Colucid, Merck, NuPathe, Eli Lilly and Company, Autonomic Technologies, Ethicon J&J, Zogenix, Supernus, Labrys, Boston Scientific, Medtronic, St Jude, Bristol-Myers Squibb, Lundbeck, Impax, MAP, Electrocore, Tonix, Novartis, Teva, Alcobra, Zosano, Insys, Ipsen, GBS/Nocira, Acorda, eNeura, Charleston Laboratories, Gore, Biohaven, Biocentric, Magellan, Theranica, Xenon, Dr. Reddy’s/Promius Pharma, Electrocore. Dr. Dodick owns equity in Epien, GBS/Nocira, Second Opinion, Healint, and Theranica. Dr. Dodick has received funding for travel, speaking, editorial activities, or royalty payments from IntraMed, SAGE Publishing, Sun Pharma, Allergan, Oxford University Press, American Academy of Neurology, American Headache Society, West Virginia University Foundation, Canadian Headache Society, Healthlogix, Universal Meeting Management, WebMD, UptoDate, Medscape, Oregon Health Science Center, Albert Einstein University, University of Toronto, Starr Clinical, Decision Resources, Synergy, MedNet LLC, Peer View Institute for Medical Education, Medicom, Chameleon Communications, Academy for Continued Healthcare Learning, Haymarket Medical Education, Global Scientific Communications, HealthLogix, Miller Medical, MeetingLogiX, Wiley Blackwell. Dr. Dodick, through his employer, has consulting use agreements with NeuroAssessment Systems and Myndshft. He holds board of director positions with King-Devick Technologies, and Epien Inc. He holds the following Patent 17,189,376.1–1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis (no compensation).
Richard Lipton receives grant support from the National Institutes of Health, the National Headache Foundation, and the Migraine Research Fund and serves as consultant, serves as an advisory board member, or has received honoraria from Alder, Allergan, American Headache Society, Autonomic Technologies, Boston Scientific, Bristol Myers Squibb, Cognimed, CoLucid, Dr. Reddy’s Laboratories/Promius Pharma, Eli Lilly and Company, eNeura Therapeutics, Merck, Novartis, Pfizer, and Teva, Inc. He receives royalties from Wolff’s Headache, 8th Edition (Oxford University Press, 2009).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Peters GA, Horton BT. Headache; With special reference to the excessive use of ergotamine tartrate and dihydroergotamine. J Lab Clin Med. 1950;36:972–973. - PubMed
-
- Headache Classification Committee of the International Headache Society (IHS) (2018) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 38(1):–211 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous