Differential insular cortex subregional vulnerability to α-synuclein pathology in Parkinson's disease and dementia with Lewy bodies
- PMID: 29797340
- PMCID: PMC7380008
- DOI: 10.1111/nan.12501
Differential insular cortex subregional vulnerability to α-synuclein pathology in Parkinson's disease and dementia with Lewy bodies
Abstract
Aim: The insular cortex consists of a heterogenous cytoarchitecture and diverse connections and is thought to integrate autonomic, cognitive, emotional and interoceptive functions to guide behaviour. In Parkinson's disease (PD) and dementia with Lewy bodies (DLB), it reveals α-synuclein pathology in advanced stages. The aim of this study is to assess the insular cortex cellular and subregional vulnerability to α-synuclein pathology in well-characterized PD and DLB subjects.
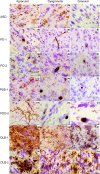
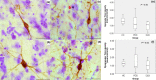
Methods: We analysed postmortem insular tissue from 24 donors with incidental Lewy body disease, PD, PD with dementia (PDD), DLB and age-matched controls. The load and distribution of α-synuclein pathology and tyrosine hydroxylase (TH) cells were studied throughout the insular subregions. The selective involvement of von Economo neurons (VENs) in the anterior insula and astroglia was assessed in all groups.
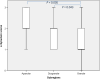
Results: A decreasing gradient of α-synuclein pathology load from the anterior periallocortical agranular towards the intermediate dysgranular and posterior isocortical granular insular subregions was found. Few VENs revealed α-synuclein inclusions while astroglial synucleinopathy was a predominant feature in PDD and DLB. TH neurons were predominant in the agranular and dysgranular subregions but did not reveal α-synuclein inclusions or significant reduction in density in patient groups.
Conclusions: Our study highlights the vulnerability of the anterior agranular insula to α-synuclein pathology in PD, PDD and DLB. Whereas VENs and astrocytes were affected in advanced disease stages, insular TH neurons were spared. Owing to the anterior insula's affective, cognitive and autonomic functions, its greater vulnerability to pathology indicates a potential contribution to nonmotor deficits in PD and DLB.
Keywords: Parkinson's disease; alpha synuclein; astrocytes; insular cortex; von Economo neurons; vulnerability.
© 2018 The Authors. Neuropathology and Applied Neurobiology published by John Wiley & Sons Ltd on behalf of British Neuropathological Society.
Figures





References
-
- Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci 2003; 991: 1–14 - PubMed
-
- Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh‐Sørensen P. Risk of dementia in Parkinson's disease: a community‐based, prospective study. Neurology 2001; 56: 730–6 - PubMed
-
- Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol 2013; 9: 13–24 - PubMed
-
- Kalia LV, Lang AE. Parkinson's disease. Lancet 2015; 386: 896–912 - PubMed
-
- McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen‐Mansfield J, Dickson D, Dubois B, Duda JE, Feldman H, Gauthier S, Halliday G, Lawlor B, Lippa C, Lopez OL, Carlos Machado J, O'Brien J, Playfer J, Reid W. International psychogeriatric association expert meeting on DLB. Dementia with Lewy bodies. Lancet Neurol 2004; 3: 19–28 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

