Spiro-epoxyglycosides as Activity-Based Probes for Glycoside Hydrolase Family 99 Endomannosidase/Endomannanase
- PMID: 29797675
- PMCID: PMC6055899
- DOI: 10.1002/chem.201801902
Spiro-epoxyglycosides as Activity-Based Probes for Glycoside Hydrolase Family 99 Endomannosidase/Endomannanase
Abstract
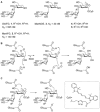
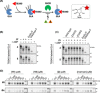
N-Glycans direct protein function, stability, folding and targeting, and influence immunogenicity. While most glycosidases that process N-glycans cleave a single sugar residue at a time, enzymes from glycoside hydrolase family 99 are endo-acting enzymes that cleave within complex N-glycans. Eukaryotic Golgi endo-1,2-α-mannosidase cleaves glucose-substituted mannose within immature glucosylated high-mannose N-glycans in the secretory pathway. Certain bacteria within the human gut microbiota produce endo-1,2-α-mannanase, which cleaves related structures within fungal mannan, as part of nutrient acquisition. An unconventional mechanism of catalysis was proposed for enzymes of this family, hinted at by crystal structures of imino/azasugars complexed within the active site. Based on this mechanism, we developed the synthesis of two glycosides bearing a spiro-epoxide at C-2 as electrophilic trap, to covalently bind a mechanistically important, conserved GH99 catalytic residue. The spiro-epoxyglycosides are equipped with a fluorescent tag, and following incubation with recombinant enzyme, allow concentration, time and pH dependent visualization of the bound enzyme using gel electrophoresis.
Keywords: GH99; activity-based probes; endomannosidase; glycosidase; inhibitors.
© 2018 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures


References
-
- Helenius A., Aebi M., Science 2001, 291, 2364. - PubMed
-
- Molinari M., Nat. Chem. Biol. 2007, 3, 313. - PubMed
-
- Feizi T., Larkin M., Glycobiology 1990, 1, 17. - PubMed
-
- Akasaka-Manya K., Manya H., Sakurai Y., Wojczyk B. S., Kozutsumi Y., Saito Y., Taniguchi N., Murayama S., Spitalnik S. L., Endo T., Glycobiology 2010, 20, 99. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

