A combined tissue-engineered/in silico signature tool patient stratification in lung cancer
- PMID: 29797762
- PMCID: PMC6068345
- DOI: 10.1002/1878-0261.12323
A combined tissue-engineered/in silico signature tool patient stratification in lung cancer
Abstract
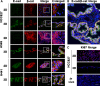
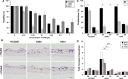
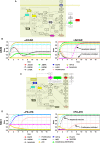
Patient-tailored therapy based on tumor drivers is promising for lung cancer treatment. For this, we combined in vitro tissue models with in silico analyses. Using individual cell lines with specific mutations, we demonstrate a generic and rapid stratification pipeline for targeted tumor therapy. We improve in vitro models of tissue conditions by a biological matrix-based three-dimensional (3D) tissue culture that allows in vitro drug testing: It correctly shows a strong drug response upon gefitinib (Gef) treatment in a cell line harboring an EGFR-activating mutation (HCC827), but no clear drug response upon treatment with the HSP90 inhibitor 17AAG in two cell lines with KRAS mutations (H441, A549). In contrast, 2D testing implies wrongly KRAS as a biomarker for HSP90 inhibitor treatment, although this fails in clinical studies. Signaling analysis by phospho-arrays showed similar effects of EGFR inhibition by Gef in HCC827 cells, under both 2D and 3D conditions. Western blot analysis confirmed that for 3D conditions, HSP90 inhibitor treatment implies different p53 regulation and decreased MET inhibition in HCC827 and H441 cells. Using in vitro data (western, phospho-kinase array, proliferation, and apoptosis), we generated cell line-specific in silico topologies and condition-specific (2D, 3D) simulations of signaling correctly mirroring in vitro treatment responses. Networks predict drug targets considering key interactions and individual cell line mutations using the Human Protein Reference Database and the COSMIC database. A signature of potential biomarkers and matching drugs improve stratification and treatment in KRAS-mutated tumors. In silico screening and dynamic simulation of drug actions resulted in individual therapeutic suggestions, that is, targeting HIF1A in H441 and LKB1 in A549 cells. In conclusion, our in vitro tumor tissue model combined with an in silico tool improves drug effect prediction and patient stratification. Our tool is used in our comprehensive cancer center and is made now publicly available for targeted therapy decisions.
Keywords: 3D lung tumor model; Boolean signaling network; HSP90 inhibitor; KRAS mutation signature; chemoresistance; in silico drug screening tool.
© 2018 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Figures




References
-
- Acquaviva J, Smith DL, Sang J, Friedland JC, He S, Sequeira M, Zhang C, Wada Y and Proia DA (2012) Targeting KRAS‐mutant non‐small cell lung cancer with the Hsp90 inhibitor ganetespib. Mol Cancer Ther 11, 2633–2643. - PubMed
-
- Alemany‐Ribes M and Semino CE (2014) Bioengineering 3D environments for cancer models. Adv Drug Deliv Rev 79–80, 40–49. - PubMed
-
- Audretsch C, Lopez D, Srivastava M, Wolz C and Dandekar T (2013) A semi‐quantitative model of Quorum‐Sensing in Staphylococcus aureus, approved by microarray meta‐analyses and tested by mutation studies. Mol BioSyst 9, 2665–2680. - PubMed
-
- Bhattacharjee Y (2012) Biomedicine. Pharma firms push for sharing of cancer trial data. Science 338, 29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

