Beyond fossil fuel-driven nitrogen transformations
- PMID: 29798857
- PMCID: PMC6088796
- DOI: 10.1126/science.aar6611
Beyond fossil fuel-driven nitrogen transformations
Abstract
Nitrogen is fundamental to all of life and many industrial processes. The interchange of nitrogen oxidation states in the industrial production of ammonia, nitric acid, and other commodity chemicals is largely powered by fossil fuels. A key goal of contemporary research in the field of nitrogen chemistry is to minimize the use of fossil fuels by developing more efficient heterogeneous, homogeneous, photo-, and electrocatalytic processes or by adapting the enzymatic processes underlying the natural nitrogen cycle. These approaches, as well as the challenges involved, are discussed in this Review.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
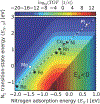
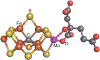
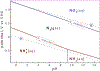
References
-
- Erisman JW, Sutton MA, Galloway J, Klimont Z, Winiwarter W, How a Century of Ammonia Synthesis Changed the World. Nat. Geosci 1, 636–639 (2008).
-
- Smil V, Detonator of the population explosion. Nature 400, 415 (1999).
-
- “Ammonia Production: Moving Towards Maximum Efficiency and Lower Ghg Emissions. ,” In Fertilizer Facts, International Fertilizer Industry Association http://www.fertilizer.org/ (2014).
-
- Booth G, Zollinger H, McLaren K, Sharples W, Westwall AE, in Ullmanns encyclopedia of industrial chemistry, Elvers B, Hawkins S, Schulz G, Eds. (2002), vol. 17.
-
- Rafiqul I, Weber C, Lehmann B, Voss A, Energy efficiency improvements in ammonia production - Perspectives and uncertainties. Energy 30, 2487–2504 (2005).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

