Tea polyphenols suppress growth and virulence-related factors of Haemophilus parasuis
- PMID: 29798967
- PMCID: PMC6068306
- DOI: 10.1292/jvms.18-0085
Tea polyphenols suppress growth and virulence-related factors of Haemophilus parasuis
Abstract
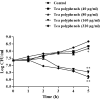
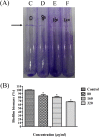
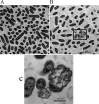
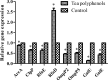
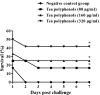
The bacterium Haemophilus parasuis (H. parasuis) is the primary cause of Glässer's disease. Currently, there are no effective vaccines that can confer protection against all H. parasuis serovars. Therefore, the present study aimed to investigate the effect of tea polyphenols on growth, expression of virulence-related factors, and biofilm formation of H. parasuis, as well as to evaluate their protective effects against H. parasuis challenge. Our findings demonstrated that tea polyphenols can inhibit H. parasuis growth in a dose-dependent manner and attenuate the biofilm formation of H. parasuis. In addition, tea polyphenols exerted inhibitory effects on the expression of H. parasuis virulence-related factors. Moreover, tea polyphenols could confer protection against a lethal dose of H. parasuis and can reduce pathological tissue damage induced by H. parasuis. In summary, our findings demonstrated the promising use of tea polyphenols as a novel treatment for H. parasuis infection in pigs.
Keywords: Haemophilus parasuis; biofilm; protection; tea polyphenols; virulence-related factor.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

