Update on glycerol-3-phosphate acyltransferases: the roles in the development of insulin resistance
- PMID: 29799006
- PMCID: PMC5968029
- DOI: 10.1038/s41387-018-0045-x
Update on glycerol-3-phosphate acyltransferases: the roles in the development of insulin resistance
Abstract
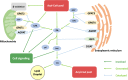
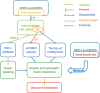
Glycerol-3-phosphate acyltransferase (GPAT) is the rate-limiting enzyme in the de novo pathway of glycerolipid synthesis. It catalyzes the conversion of glycerol-3-phosphate and long-chain acyl-CoA to lysophosphatidic acid. In mammals, four isoforms of GPATs have been identified based on subcellular localization, substrate preferences, and NEM sensitivity, and they have been classified into two groups, one including GPAT1 and GPAT2, which are localized in the mitochondrial outer membrane, and the other including GPAT3 and GPAT4, which are localized in the endoplasmic reticulum membrane. GPATs play a pivotal role in the regulation of triglyceride and phospholipid synthesis. Through gain-of-function and loss-of-function experiments, it has been confirmed that GPATs play a critical role in the development of obesity, hepatic steatosis, and insulin resistance. In line with this, the role of GPATs in metabolism was supported by studies using a GPAT inhibitor, FSG67. Additionally, the functional characteristics of GPATs and the relation between three isoforms (GPAT1, 3, and 4) and insulin resistance has been described in this review.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Glycerol-3-phosphate acyltransferases and metabolic syndrome: recent advances and future perspectives.Expert Rev Mol Med. 2022 Sep 5;24:e30. doi: 10.1017/erm.2022.23. Expert Rev Mol Med. 2022. PMID: 36059117 Review.
-
Thematic review series: glycerolipids. Mammalian glycerol-3-phosphate acyltransferases: new genes for an old activity.J Lipid Res. 2008 Oct;49(10):2079-88. doi: 10.1194/jlr.R800013-JLR200. Epub 2008 Jul 24. J Lipid Res. 2008. PMID: 18658143 Review.
-
Transcriptional Regulation of Acyl-CoA:Glycerol-sn-3-Phosphate Acyltransferases.Int J Mol Sci. 2019 Feb 22;20(4):964. doi: 10.3390/ijms20040964. Int J Mol Sci. 2019. PMID: 30813330 Free PMC article. Review.
-
[Sn-glycerol-3-phosphate acyltransferases (GPATs) in plants].Yi Chuan. 2013 Dec;35(12):1352-9. doi: 10.3724/sp.j.1005.2013.01352. Yi Chuan. 2013. PMID: 24645344 Review. Chinese.
-
Regulation of Triglyceride Metabolism. II. Function of mitochondrial GPAT1 in the regulation of triacylglycerol biosynthesis and insulin action.Am J Physiol Gastrointest Liver Physiol. 2007 May;292(5):G1195-9. doi: 10.1152/ajpgi.00553.2006. Epub 2006 Dec 7. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17158253 Free PMC article. Review.
Cited by
-
Diacylglycerol Kinases and Its Role in Lipid Metabolism and Related Diseases.Int J Mol Sci. 2024 Dec 9;25(23):13207. doi: 10.3390/ijms252313207. Int J Mol Sci. 2024. PMID: 39684917 Free PMC article. Review.
-
Progress of Conjugated Linoleic Acid on Milk Fat Metabolism in Ruminants and Humans.Animals (Basel). 2023 Nov 6;13(21):3429. doi: 10.3390/ani13213429. Animals (Basel). 2023. PMID: 37958184 Free PMC article. Review.
-
The Role of Dietary and Microbial Fatty Acids in the Control of Inflammation in Neonatal Piglets.Animals (Basel). 2021 Sep 24;11(10):2781. doi: 10.3390/ani11102781. Animals (Basel). 2021. PMID: 34679802 Free PMC article. Review.
-
New Mammalian Glycerol-3-Phosphate Phosphatase: Role in β-Cell, Liver and Adipocyte Metabolism.Front Endocrinol (Lausanne). 2021 Jul 13;12:706607. doi: 10.3389/fendo.2021.706607. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34326816 Free PMC article. Review.
-
Rapamycin/metformin co-treatment normalizes insulin sensitivity and reduces complications of metabolic syndrome in type 2 diabetic mice.Aging Cell. 2022 Sep;21(9):e13666. doi: 10.1111/acel.13666. Epub 2022 Aug 19. Aging Cell. 2022. PMID: 35986566 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

