Cost-Effectiveness of Ribociclib plus Letrozole Versus Palbociclib plus Letrozole and Letrozole Monotherapy in the First-Line Treatment of Postmenopausal Women with HR+/HER2- Advanced or Metastatic Breast Cancer: A U.S. Payer Perspective
- PMID: 29799329
- PMCID: PMC10398120
- DOI: 10.18553/jmcp.2018.24.6.514
Cost-Effectiveness of Ribociclib plus Letrozole Versus Palbociclib plus Letrozole and Letrozole Monotherapy in the First-Line Treatment of Postmenopausal Women with HR+/HER2- Advanced or Metastatic Breast Cancer: A U.S. Payer Perspective
Abstract
Background: U.S. regulatory approvals of the cyclin-dependent kinase 4 and 6 (CDK 4/6) inhibitors ribociclib and palbociclib as add-ons to letrozole greatly enhance the prospects for treating postmenopausal women with hormone receptor-positive (HR+)/human epidermal receptor 2-negative (HER2-) advanced or metastatic breast cancer. Clinical trials have established that the combination of a CDK 4/6 inhibitor with letrozole can significantly improve progression-free survival (PFS) versus letrozole monotherapy and is safe and well tolerated. Cost-effectiveness studies are required to inform payers and clinical decision makers on the money value of combination treatment in clinical practice.
Objective: To evaluate the cost-effectiveness of ribociclib plus letrozole versus palbociclib plus letrozole and versus letrozole monotherapy in the first-line treatment of postmenopausal women with HR+/HER2- advanced or metastatic breast cancer from a U.S. private third-party payer perspective.
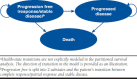
Methods: A partitioned survival model including 3 health states (progression free, with either overall response or stable disease; progressed disease; and death) simulated lifetime costs and outcomes over a 40-year lifetime horizon with a 1-month cycle length. Clinical efficacy data (PFS and overall survival [OS]) were derived from a phase III trial of ribociclib plus letrozole (MONALEESA-2; NCT01958021), a phase II trial of palbociclib plus letrozole (PALOMA-1; NCT00721409), and a Bayesian network meta-analysis. Health care costs included drug acquisition and monitoring, disease management, subsequent therapies, and serious drug-related adverse events. Effectiveness was measured in life-years, derived from survival projections, and in quality-adjusted life-years (QALYs), calculated from time spent in each state combined with health-state utility values. A one-way deterministic sensitivity analysis explored the impact of uncertainty in key model parameters on results, and probabilistic uncertainty was assessed through a Monte Carlo probabilistic sensitivity analysis.
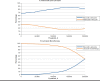
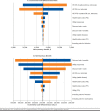
Results: Ribociclib plus letrozole was dominant versus palbociclib plus letrozole, with a cost saving of $43,037 and a gain of 0.086 QALYs. Compared with letrozole monotherapy, ribociclib plus letrozole was associated with an incremental cost of $144,915 and an incremental QALY of 0.689, equating to an incremental cost-effectiveness ratio of $210,369 per QALY. Key model drivers included OS HRs for palbociclib plus letrozole versus letrozole and for ribociclib plus letrozole versus letrozole, the PFS HR for palbociclib plus letrozole versus letrozole, PD health-state costs, utility of response, and cost discount rate. The probabilities that ribociclib plus letrozole was cost-effective versus letrozole at thresholds of $50,000, $100,000 and $200,000 per QALY gained were 1.6%, 6.3%, and 50.5%, respectively.
Conclusions: In the United States, ribociclib plus letrozole is a cost-effective alternative to palbociclib plus letrozole for the first-line treatment of postmenopausal women with HR+/HER2- advanced or metastatic breast cancer. Ribociclib plus letrozole is also cost-effective versus letrozole monotherapy at willingness-to-pay thresholds greater than $198,000 per QALY (for probabilistic analysis).
Disclosures: Funding for this study was provided by Novartis, which manufactures ribociclib and provided input on the study design and data collection, analysis, and interpretation. Mistry, May, Suri, and Young are employees of PAREXEL. Tang, Mishra, D. Bhattacharyya, and Dalal are employees of Novartis. S. Bhattacharyya was an employee of Novartis during the study period. Tang and Dalal hold stock in Novartis. Brixner, Oderda, and Biskupiak were paid by Millcreek Outcomes Group as consultants for work on this project. Brixner has also consulted for AstraZeneca, UCB, Regeneron, and Abbott.
Conflict of interest statement
Funding for this study was provided by Novartis, which manufactures ribociclib and provided input on the study design and data collection, analysis, and interpretation. Mistry, May, Suri, and Young are employees of PAREXEL. Tang, Mishra, D. Bhattacharyya, and Dalal are employees of Novartis. S. Bhattacharyya was an employee of Novartis during the study period. Tang and Dalal hold stock in Novartis. Brixner, Oderda, and Biskupiak were paid by Millcreek Outcomes Group as consultants for work on this project. Brixner has also consulted for AstraZeneca, UCB, Regeneron, and Abbott.
Figures
Similar articles
-
Cost-effectiveness of ribociclib plus endocrine therapy versus placebo plus endocrine therapy in HR-positive, HER2-negative breast cancer.J Manag Care Spec Pharm. 2021 Mar;27(3):327-338. doi: 10.18553/jmcp.2021.27.3.327. J Manag Care Spec Pharm. 2021. PMID: 33645243 Free PMC article.
-
Quantification of Economic Impact of Drug Wastage in Oral Oncology Medications: Comparison of 3 Methods Using Palbociclib and Ribociclib in Advanced or Metastatic Breast Cancer.J Manag Care Spec Pharm. 2019 Aug;25(8):859-866. doi: 10.18553/jmcp.2019.25.8.859. J Manag Care Spec Pharm. 2019. PMID: 31347980 Free PMC article.
-
Cost-effectiveness of ribociclib versus palbociclib in combination with an aromatase inhibitor as first-line treatment of postmenopausal women with HR+/HER2- advanced breast cancer: analysis based on final OS results of MONALEESA-2 and PALOMA-2.J Med Econ. 2023 Jan-Dec;26(1):357-365. doi: 10.1080/13696998.2023.2182051. J Med Econ. 2023. PMID: 36797664
-
Ribociclib with an Aromatase Inhibitor for Previously Untreated, HR-Positive, HER2-Negative, Locally Advanced or Metastatic Breast Cancer: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2019 Feb;37(2):141-153. doi: 10.1007/s40273-018-0708-4. Pharmacoeconomics. 2019. PMID: 30194622 Free PMC article. Review.
-
Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis.Lancet Oncol. 2019 Oct;20(10):1360-1369. doi: 10.1016/S1470-2045(19)30420-6. Epub 2019 Sep 4. Lancet Oncol. 2019. PMID: 31494037
Cited by
-
Cost-effectiveness analysis of trastuzumab deruxtecan versus trastuzumab emtansine for HER2-positive breast cancer.Front Pharmacol. 2022 Sep 9;13:924126. doi: 10.3389/fphar.2022.924126. eCollection 2022. Front Pharmacol. 2022. PMID: 36160459 Free PMC article.
-
Cost-effectiveness of Ribociclib in HER2- negative breast cancer: A synthesis of current evidence.Saudi Pharm J. 2022 Aug;30(8):1113-1119. doi: 10.1016/j.jsps.2022.06.002. Epub 2022 Jun 13. Saudi Pharm J. 2022. PMID: 36164576 Free PMC article.
-
CDK4/6 Inhibitors in the First-Line Treatment of Postmenopausal Women with HR+/HER2- Advanced or Metastatic Breast Cancer: An Updated Network Meta-Analysis and Cost-Effectiveness Analysis.Cancers (Basel). 2023 Jun 28;15(13):3386. doi: 10.3390/cancers15133386. Cancers (Basel). 2023. PMID: 37444496 Free PMC article.
-
Economic Evaluation of Sacituzumab Govitecan for the Treatment of Metastatic Triple-Negative Breast Cancer in China and the US.Front Oncol. 2021 Oct 28;11:734594. doi: 10.3389/fonc.2021.734594. eCollection 2021. Front Oncol. 2021. PMID: 34778047 Free PMC article.
-
Cost-Effectiveness Analysis of Atezolizumab Plus Chemotherapy in the First-Line Treatment of Metastatic Non-Squamous Non-Small Cell Lung Cancer.Adv Ther. 2020 May;37(5):2116-2126. doi: 10.1007/s12325-020-01292-3. Epub 2020 Mar 19. Adv Ther. 2020. PMID: 32193809
References
-
- Gradishar WJ, Anderson BO, Balassanian R, et al. . Invasive breast cancer version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(3):324-54. - PubMed
-
- Cardoso F, Costa A, Norton L, et al. . ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast. 2014;23(5):489-502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous