The ABP Dendrimer, a Drug-Candidate against Inflammatory Diseases That Triggers the Activation of Interleukin-10 Producing Immune Cells
- PMID: 29799517
- PMCID: PMC6100262
- DOI: 10.3390/molecules23061272
The ABP Dendrimer, a Drug-Candidate against Inflammatory Diseases That Triggers the Activation of Interleukin-10 Producing Immune Cells
Abstract
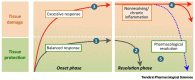
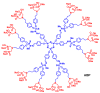
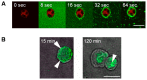
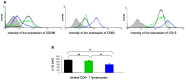
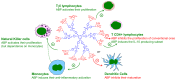
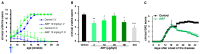
The ABP dendrimer, which is built on a phosphorus-based scaffold and bears twelve azabisphosphonate groups at its surface, is one of the dendrimers that has been shown to display immuno-modulatory and anti-inflammatory effects towards the human immune system. Its anti-inflammatory properties have been successfully challenged in animal models of inflammatory disorders. In this review, we trace the discovery and the evaluation of the therapeutic effects of the ABP dendrimer in three different animal models of both acute and chronic inflammatory diseases. We emphasize that its therapeutic effects rely on the enhancement of the production of Interleukin-10, the paradigm of anti-inflammatory cytokines, by different subsets of immune cells, such as monocytes/macrophages and CD4+ T lymphocytes.
Keywords: azabisphosphonate; chronic inflammatory diseases; inflammation; interleukin-10; monocytes/macrophages; phosphorus-based dendrimers; resolution.
Conflict of interest statement
R.P. is co-founder and shareholder of IMD-Pharma.
Figures

Similar articles
-
Phosphorus-Based Dendrimer ABP Treats Neuroinflammation by Promoting IL-10-Producing CD4(+) T Cells.Biomacromolecules. 2015 Nov 9;16(11):3425-33. doi: 10.1021/acs.biomac.5b00643. Epub 2015 Oct 7. Biomacromolecules. 2015. PMID: 26397709
-
An azabisphosphonate-capped poly(phosphorhydrazone) dendrimer for the treatment of endotoxin-induced uveitis.Molecules. 2013 Aug 5;18(8):9305-16. doi: 10.3390/molecules18089305. Molecules. 2013. PMID: 23921793 Free PMC article.
-
Interaction studies reveal specific recognition of an anti-inflammatory polyphosphorhydrazone dendrimer by human monocytes.Nanoscale. 2015 Nov 14;7(42):17672-84. doi: 10.1039/c5nr03884g. Nanoscale. 2015. PMID: 26335052
-
Curing inflammatory diseases using phosphorous dendrimers.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022 Jul;14(4):e1783. doi: 10.1002/wnan.1783. Epub 2022 Feb 22. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022. PMID: 35194953 Review.
-
Regulatory B cells in human inflammatory and autoimmune diseases: from mouse models to clinical research.Int Immunol. 2015 Oct;27(10):495-504. doi: 10.1093/intimm/dxv026. Epub 2015 May 8. Int Immunol. 2015. PMID: 25957264 Review.
Cited by
-
An Anti-Inflammatory Poly(PhosphorHydrazone) Dendrimer Capped with AzaBisPhosphonate Groups to Treat Psoriasis.Biomolecules. 2020 Jun 23;10(6):949. doi: 10.3390/biom10060949. Biomolecules. 2020. PMID: 32586038 Free PMC article.
-
Multivalent nanosystems: targeting monocytes/macrophages.Int J Nanomedicine. 2018 Sep 19;13:5511-5521. doi: 10.2147/IJN.S146192. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30271144 Free PMC article. Review.
-
Biodistribution and Biosafety of a Poly(Phosphorhydrazone) Dendrimer, an Anti-Inflammatory Drug-Candidate.Biomolecules. 2019 Sep 11;9(9):475. doi: 10.3390/biom9090475. Biomolecules. 2019. PMID: 31514434 Free PMC article.
-
Dendrimers and Derivatives as Multifunctional Nanotherapeutics for Alzheimer's Disease.Pharmaceutics. 2023 Mar 24;15(4):1054. doi: 10.3390/pharmaceutics15041054. Pharmaceutics. 2023. PMID: 37111540 Free PMC article. Review.
-
Repolarization of Unbalanced Macrophages: Unmet Medical Need in Chronic Inflammation and Cancer.Int J Mol Sci. 2022 Jan 28;23(3):1496. doi: 10.3390/ijms23031496. Int J Mol Sci. 2022. PMID: 35163420 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

