Exploring Applications of Covalent Organic Frameworks: Homogeneous Reticulation of Radicals for Dynamic Nuclear Polarization
- PMID: 29799739
- PMCID: PMC6045815
- DOI: 10.1021/jacs.8b02839
Exploring Applications of Covalent Organic Frameworks: Homogeneous Reticulation of Radicals for Dynamic Nuclear Polarization
Abstract
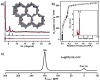
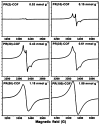
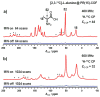
Rapid progress has been witnessed in the past decade in the fields of covalent organic frameworks (COFs) and dynamic nuclear polarization (DNP). In this contribution, we bridge these two fields by constructing radical-embedded COFs as promising DNP agents. Via polarization transfer from unpaired electrons to nuclei, DNP realizes significant enhancement of NMR signal intensities. One of the crucial issues in DNP is to screen for suitable radicals to act as efficient polarizing agents, the basic criteria for which are homogeneous distribution and fixed orientation of unpaired electrons. We therefore envisioned that the crystalline and porous structures of COFs, if evenly embedded with radicals, may work as a new "crystalline sponge" for DNP experiments. As a proof of concept, we constructed a series of proxyl-radical-embedded COFs (denoted as PR( x)-COFs) and successfully applied them to achieve substantial DNP enhancement. Benefiting from the bottom-up and multivariate synthetic strategies, proxyl radicals have been covalently reticulated, homogeneously distributed, and rigidly embedded into the crystalline and mesoporous frameworks with adjustable concentration ( x%). Excellent performance of PR( x)-COFs has been observed for DNP 1H, 13C, and 15N solid-state NMR enhancements. This contribution not only realizes the direct construction of radical COFs from radical monomers, but also explores the new application of COFs as DNP polarizing agents. Given that many radical COFs can therefore be rationally designed and facilely constructed with well-defined composition, distribution, and pore size, we expect that our effort will pave the way for utilizing radical COFs as standard polarizing agents in DNP NMR experiments.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Côté AP, Benin AI, Ockwig NW, O’Keeffe M, Matzger AJ, Yaghi OM. Science. 2005;310:1166. - PubMed
-
-
For reviews, see: Feng X, Ding X, Jiang D. Chem Soc Rev. 2012;41:6010.Ding SY, Wang W. Chem Soc Rev. 2013;42:548.Colson JW, Dichtel WR. Nat Chem. 2013;5:453.Waller PJ, Gándara F, Yaghi OM. Acc Chem Res. 2015;48:3053.Slater AG, Cooper AI. Science. 2015;348:aaa8075.Huang N, Wang P, Jiang D. Nat Rev Mater. 2016;1:16068.Segura JL, Mancheno MJ, Zamora F. Chem Soc Rev. 2016;45:5635.Jiang J, Zhao Y, Yaghi OM. J Am Chem Soc. 2016;138:3255.Diercks CS, Yaghi OM. Science. 2017;355:923.Rogge SMJ, Bavykina A, Hajek J, Garcia H, Olivos-Suarez AI, Sepulveda-Escribano A, Vimont A, Clet G, Bazin P, Kapteijn F, Daturi M, Ramos-Fernandez EV, Llabres i Xamena FX, Van Speybroeck V, Gascon J. Chem Soc Rev. 2017;46:3134.Jin Y, Hu Y, Zhang W. Nat Rev Chem. 2017;1:0056.Beuerle F, Gole B. Angew Chem Int Ed. 2018;57:4850.
-
-
-
A more comprehensive list of the references has been presented in SI. For selected examples, see: Han SS, Furukawa H, Yaghi OM, Goddard WA. J Am Chem Soc. 2008;130:11580.Ding SY, Gao J, Wang Q, Zhang Y, Song WG, Su CY, Wang W. J Am Chem Soc. 2011;133:19816.Ding X, Guo J, Feng X, Honsho Y, Guo J, Seki S, Maitarad P, Saeki A, Nagase S, Jiang D. Angew Chem Int Ed. 2011;50:1289.Yu JT, Chen Z, Sun J, Huang ZT, Zheng QY. J Mater Chem. 2012;22:5369.DeBlase CR, Silberstein KE, Truong TT, Abruña HD, Dichtel WR. J Am Chem Soc. 2013;135:16821.Bertrand GHV, Michaelis VK, Ong TC, Griffin RG, Dincã M. Proc Natl Acad Sci USA. 2013;110:4923.Dogru M, Handloser M, Auras F, Kunz T, Medina D, Hartschuh A, Knochel P, Bein T. Angew Chem Int Ed. 2013;125:2992.Chandra S, Kundu T, Kandambeth S, BabaRao R, Marathe Y, Kunjir SM, Banerjee R. J Am Chem Soc. 2014;136:6570.Fang Q, Wang J, Gu S, Kaspar RB, Zhuang Z, Zheng J, Guo H, Qiu S, Yan Y. J Am Chem Soc. 2015;137:8352.Wang X, Han X, Zhang J, Wu X, Liu Y, Cui Y. J Am Chem Soc. 2016;138:12332.Sun Q, Aguila B, Perman J, Nguyen N, Ma S. J Am Chem Soc. 2016;138:15790.Ma H, Liu B, Li B, Zhang L, Li YG, Tan HQ, Zang HY, Zhu G. J Am Chem Soc. 2016;138:5897.Yin ZJ, Xu SQ, Zhan TG, Qi QY, Wu ZQ, Zhao X. Chem Commun. 2017;53:7266.Lu S, Hu Y, Wan S, McCaffrey R, Jin Y, Gu H, Zhang W. J Am Chem Soc. 2017;139:17082.Banerjee T, Haase F, Savasci G, Gottschling K, Ochsenfeld C, Lotsch BV. J Am Chem Soc. 2017;139:16228.Li Z, Li H, Guan X, Tang J, Yusran Y, Li Z, Xue M, Fang Q, Yan Y, Valtchev V, Qiu S. J Am Chem Soc. 2017;139:17771.
-
-
-
For the earliest reports, see: Overhauser AW. Phys Review. 1953;92:411.Carver TR, Slichter CP. Phys Rev. 1953;92:212.. For selected reviews, see: Morley GW, van Tol J, Ardavan A, Porfyrakis K, Zhang J, Briggs GA. Phys Rev Lett. 2007;98:220501.Maly T, Debelouchina GT, Bajaj VS, Hu KN, Joo CG, Mak-Jurkauskas ML, Sirigiri JR, van der Wel PC, Herzfeld J, Temkin RJ, Griffin RG. J Chem Phys. 2008;128:052211.Griesinger C, Bennati M, Vieth HM, Luchinat C, Parigi G, Hofer P, Engelke F, Glaser SJ, Denysenkov V, Prisner TF. Prog Nucl Magn Reson Spectrosc. 2012;64:4.Hovav Y, Feintuch A, Vega S. J Magn Reson. 2012;214:29.Rossini AJ, Zagdoun A, Lelli M, Lesage A, Copéret C, Emsley L. Acc Chem Res. 2013;46:1942.Franck JM, Pavlova A, Scott JA, Han S. Prog Nucl Magn Reson Spectrosc. 2013;74:33.Keshari KR, Wilson DM. Chem Soc Rev. 2014;43:1627.Kobayashi T, Perras FA, Slowing II, Sadow AD, Pruski M. ACS Catal. 2015;5:7055.Lee D, Hediger S, De Paëpe G. Solid State Nucl Magn Reson. 2015;66–67:6.Smith AN, Long JR. Anal Chem. 2016;88:122.Lilly Thankamony AS, Wittmann JJ, Kaushik M, Corzilius B. Prog Nucl Magn Reson Spectrosc. 2017;102–103:120.
-
-
-
A more comprehensive list of the references has been presented in SI. For selected examples, see: Prandolini MJ, Denysenkov VP, Gafurov M, Endeward B, Prisner TF. J Am Chem Soc. 2009;131:6090.Salnikov E, Rosay M, Pawsey S, Ouari O, Tordo P, Bechinger B. J Am Chem Soc. 2010;132:5940.Corzilius B, Smith AA, Barnes AB, Luchinat C, Bertini I, Griffin RG. J Am Chem Soc. 2011;133:5648.Lee D, Takahashi H, Thankamony AS, Dacquin JP, Bardet M, Lafon O, Paepe GD. J Am Chem Soc. 2012;134:18491.Blanc F, Sperrin L, Jefferson DA, Pawsey S, Rosay M, Grey CP. J Am Chem Soc. 2013;135:2975.Wolf P, Valla M, Rossini AJ, Comas-Vives A, Nunez-Zarur F, Malaman B, Lesage A, Emsley L, Coperet C, Hermans I. Angew Chem Int Ed. 2014;53:10179.Valla M, Rossini AJ, Caillot M, Chizallet C, Raybaud P, Digne M, Chaumonnot A, Lesage A, Emsley L, van Bokhoven JA, Coperet C. J Am Chem Soc. 2015;137:10710.Lilly Thankamony AS, Lion C, Pour-point F, Singh B, Perez Linde AJ, Carnevale D, Bodenhausen G, Vezin H, Lafon O, Polshettiwar V. Angew Chem Int Ed. 2015;54:2190.Perras FA, Kobayashi T, Pruski M. J Am Chem Soc. 2015;137:8336.Mollica G, Dekhil M, Ziarelli F, Thureau P, Viel S. Angew Chem Int Ed. 2015;54:6028.Mouat AR, George C, Kobayashi T, Pruski M, van Duyne RP, Marks TJ, Stair PC. Angew Chem Int Ed. 2015;54:13346.Potapov A, Yau WM, Ghirlando R, Thurber KR, Tycko R. J Am Chem Soc. 2015;137:8294.Kaplan M, Narasimhan S, de Heus C, Mance D, van Doorn S, Houben K, Popov-Celeketic D, Damman R, Katrukha EA, Jain P, Geerts WJC, Heck AJR, Folkers GE, Kapitein LC, Lemeer S, van Bergen En Henegouwen PMP, Baldus M. Cell. 2016;167:1241.Stoppler D, Song C, van Rossum BJ, Geiger MA, Lang C, Mroginski MA, Jagtap AP, Sigurdsson ST, Matysik J, Hughes J, Oschkinat H. Angew Chem Int Ed. 2016;55:16017.Kaminker I, Shimon D, Hovav Y, Feintuch A, Vega S. Phys Chem Chem Phys. 2016;18:11017.Ji X, Bornet A, Vuichoud B, Milani J, Gajan D, Rossini AJ, Emsley L, Bodenhausen G, Jannin S. Nat Commun. 2017;8:13975.Berruyer P, Lelli M, Conley MP, Silverio DL, Widdifield CM, Siddiqi G, Gajan D, Lesage A, Coperet C, Emsley L. J Am Chem Soc. 2017;139:849.Saliba EP, Sesti EL, Scott FJ, Albert BJ, Choi EJ, Alaniva N, Gao C, Barnes AB. J Am Chem Soc. 2017;139:6310.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

