Effect of More vs Less Frequent Follow-up Testing on Overall and Colorectal Cancer-Specific Mortality in Patients With Stage II or III Colorectal Cancer: The COLOFOL Randomized Clinical Trial
- PMID: 29800179
- PMCID: PMC6583244
- DOI: 10.1001/jama.2018.5623
Effect of More vs Less Frequent Follow-up Testing on Overall and Colorectal Cancer-Specific Mortality in Patients With Stage II or III Colorectal Cancer: The COLOFOL Randomized Clinical Trial
Abstract
Importance: Intensive follow-up of patients after curative surgery for colorectal cancer is common in clinical practice, but evidence of a survival benefit is limited.
Objective: To examine overall mortality, colorectal cancer-specific mortality, and colorectal cancer-specific recurrence rates among patients with stage II or III colorectal cancer who were randomized after curative surgery to 2 alternative schedules for follow-up testing with computed tomography and carcinoembryonic antigen.
Design, setting, and participants: Unblinded randomized trial including 2509 patients with stage II or III colorectal cancer treated at 24 centers in Sweden, Denmark, and Uruguay from January 2006 through December 2010 and followed up for 5 years; follow-up ended on December 31, 2015.
Interventions: Patients were randomized either to follow-up testing with computed tomography of the thorax and abdomen and serum carcinoembryonic antigen at 6, 12, 18, 24, and 36 months after surgery (high-frequency group; n = 1253 patients) or at 12 and 36 months after surgery (low-frequency group; n = 1256 patients).
Main outcomes and measures: The primary outcomes were 5-year overall mortality and colorectal cancer-specific mortality rates. The secondary outcome was the colorectal cancer-specific recurrence rate. Both intention-to-treat and per-protocol analyses were performed.
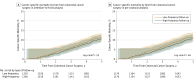
Results: Among 2555 patients who were randomized, 2509 were included in the intention-to-treat analysis (mean age, 63.5 years; 1128 women [45%]) and 2365 (94.3%) completed the trial. The 5-year overall patient mortality rate in the high-frequency group was 13.0% (161/1253) compared with 14.1% (174/1256) in the low-frequency group (risk difference, 1.1% [95% CI, -1.6% to 3.8%]; P = .43). The 5-year colorectal cancer-specific mortality rate in the high-frequency group was 10.6% (128/1248) compared with 11.4% (137/1250) in the low-frequency group (risk difference, 0.8% [95% CI, -1.7% to 3.3%]; P = .52). The colorectal cancer-specific recurrence rate was 21.6% (265/1248) in the high-frequency group compared with 19.4% (238/1250) in the low-frequency group (risk difference, 2.2% [95% CI, -1.0% to 5.4%]; P = .15).
Conclusions and relevance: Among patients with stage II or III colorectal cancer, follow-up testing with computed tomography and carcinoembryonic antigen more frequently compared with less frequently did not result in a significant rate reduction in 5-year overall mortality or colorectal cancer-specific mortality.
Trial registration: clinicaltrials.gov Identifier: NCT00225641.
Conflict of interest statement
Figures



Comment in
-
Best Evidence Supports Annual Surveillance for Resected Colorectal Cancer.JAMA. 2018 May 22;319(20):2083-2085. doi: 10.1001/jama.2018.5817. JAMA. 2018. PMID: 29800158 No abstract available.
-
After surgery for stage II or III colorectal cancer, more vs less frequent follow-up did not differ for 5-year mortality.Ann Intern Med. 2018 Oct 16;169(8):JC38. doi: 10.7326/ACPJC-2018-169-8-038. Ann Intern Med. 2018. PMID: 30326082 No abstract available.
References
-
- GLOBOCAN 2012 fact sheet. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. Accessed April 14, 2018.
-
- Maringe C, Walters S, Rachet B, et al. . Stage at diagnosis and colorectal cancer survival in six high-income countries. Acta Oncol. 2013;52(5):919-932. - PubMed
-
- Steele SR, Chang GJ, Hendren S, et al. . Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58(8):713-725. - PubMed
-
- Rosen M, Chan L, Beart RW Jr, Vukasin P, Anthone G. Follow-up of colorectal cancer. Dis Colon Rectum. 1998;41(9):1116-1126. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

