Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide
- PMID: 29800237
- PMCID: PMC6106108
- DOI: 10.1093/cvr/cvy112
Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide
Abstract
Aims: Monocytes play an important role in hypertension. Circulating monocytes in humans exist as classical, intermediate, and non-classical forms. Monocyte differentiation can be influenced by the endothelium, which in turn is activated in hypertension by mechanical stretch. We sought to examine the role of increased endothelial stretch and hypertension on monocyte phenotype and function.
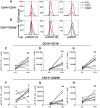
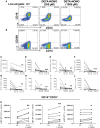
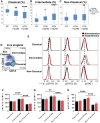
Methods and results: Human monocytes were cultured with confluent human aortic endothelial cells undergoing either 5% or 10% cyclical stretch. We also characterized circulating monocytes in normotensive and hypertensive humans. In addition, we quantified accumulation of activated monocytes and monocyte-derived cells in aortas and kidneys of mice with Angiotensin II-induced hypertension. Increased endothelial stretch enhanced monocyte conversion to CD14++CD16+ intermediate monocytes and monocytes bearing the CD209 marker and markedly stimulated monocyte mRNA expression of interleukin (IL)-6, IL-1β, IL-23, chemokine (C-C motif) ligand 4, and tumour necrosis factor α. STAT3 in monocytes was activated by increased endothelial stretch. Inhibition of STAT3, neutralization of IL-6 and scavenging of hydrogen peroxide prevented formation of intermediate monocytes in response to increased endothelial stretch. We also found evidence that nitric oxide (NO) inhibits formation of intermediate monocytes and STAT3 activation. In vivo studies demonstrated that humans with hypertension have increased intermediate and non-classical monocytes and that intermediate monocytes demonstrate evidence of STAT3 activation. Mice with experimental hypertension exhibit increased aortic and renal infiltration of monocytes, dendritic cells, and macrophages with activated STAT3.
Conclusions: These findings provide insight into how monocytes are activated by the vascular endothelium during hypertension. This is likely in part due to a loss of NO signalling and increased release of IL-6 and hydrogen peroxide by the dysfunctional endothelium and a parallel increase in STAT activation in adjacent monocytes. Interventions to enhance bioavailable NO, reduce IL-6 or hydrogen peroxide production or to inhibit STAT3 may have anti-inflammatory roles in hypertension and related conditions.
Figures





Comment in
-
Mechanical stretch on endothelial cells interconnects innate and adaptive immune response in hypertension.Cardiovasc Res. 2018 Sep 1;114(11):1432-1434. doi: 10.1093/cvr/cvy148. Cardiovasc Res. 2018. PMID: 29912294 No abstract available.
References
-
- Global Burden of Disease Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1659–1724. - PMC - PubMed
-
- Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Munzel T.. Lysozyme M-positive monocytes mediate Angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 2011;124:1370–1381. - PubMed
-
- De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL.. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol 2005;25:2106–2113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

