Dengue seroprevalence: data from the clinical development of a tetravalent dengue vaccine in 14 countries (2005-2014)
- PMID: 29800279
- PMCID: PMC5972646
- DOI: 10.1093/trstmh/try037
Dengue seroprevalence: data from the clinical development of a tetravalent dengue vaccine in 14 countries (2005-2014)
Abstract
Dengue seroprevalence data in the literature is limited and the available information is difficult to compare between studies because of the varying survey designs and methods used. We assessed dengue seropositivity across 14 countries using data from 15 trials conducted during the development of a tetravalent dengue vaccine between October 2005 and February 2014. Participants' dengue seropositivity (n=8592) was determined from baseline (before vaccination) serum samples at two centralized laboratories with the plaque reduction neutralization test (PRNT50). Seropositivity rates generally increased with age in endemic settings. Although seropositivity rates varied across geographical areas, between countries, and within countries by region, no major differences were observed for given age groups between the two endemic regions, Latin America and Asia-Pacific. Seropositivity rates were generally stable over time. The proportion of participants who had only experienced primary infection tended to be higher in younger children than adolescents/adults. These results will help inform and guide dengue control strategies in the participating countries.
Figures
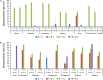
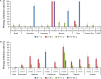
References
-
- World Health Organization Dengue: guidelines for treatment, prevention and control. 2009. http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf [accessed 21 March 2017]. - PubMed
-
- Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine 2012;30(29):4301–6. - PubMed
-
- Simmons CP, Farrar JJ, Nguyen vV et al. . Dengue. N Engl J Med 2012;366(15):1423–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

