Human Experimental Challenge With Enterotoxigenic Escherichia coli Elicits Immune Responses to Canonical and Novel Antigens Relevant to Vaccine Development
- PMID: 29800314
- PMCID: PMC6151082
- DOI: 10.1093/infdis/jiy312
Human Experimental Challenge With Enterotoxigenic Escherichia coli Elicits Immune Responses to Canonical and Novel Antigens Relevant to Vaccine Development
Abstract
Background: Enterotoxigenic Escherichia coli (ETEC) is a major cause of diarrheal illness in the developing world. Enterotoxigenic E coli vaccinology has been challenged by genetic diversity and heterogeneity of canonical antigens. Examination of the antigenic breadth of immune responses associated with protective immunity could afford new avenues for vaccine development.
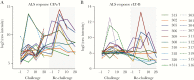
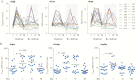
Methods: Antibody lymphocyte supernatants (ALS) and sera from 20 naive human volunteers challenged with ETEC strain H10407 and from 10 volunteers rechallenged 4-6 weeks later with the same strain (9 of whom were completely protected on rechallenge) were tested against ETEC proteome microarrays containing 957 antigens.
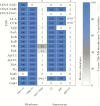
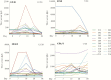
Results: Enterotoxigenic E coli challenge stimulated robust serum and mucosal (ALS) responses to canonical vaccine antigens (CFA/I, and the B subunit of LT) as well as a small number of antigens not presently targeted in ETEC vaccines. These included pathovar-specific secreted proteins (EtpA, EatA) as well as highly conserved E coli antigens including YghJ, flagellin, and pertactin-like autotransporter proteins, all of which have previously afforded protection against ETEC infection in preclinical studies.
Conclusions: Taken together, studies reported here suggest that immune responses after ETEC infection involve traditional vaccine targets as well as a select number of more recently identified protein antigens that could offer additional avenues for vaccine development for these pathogens.
Figures

References
-
- Kotloff KL, Nataro JP, Blackwelder WC, et al. . Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. - PubMed
-
- Kotloff KL, Platts-Mills JA, Nasrin D, Roose A, Blackwelder WC, Levine MM. Global burden of diarrheal diseases among children in developing countries: incidence, etiology, and insights from new molecular diagnostic techniques. Vaccine 2017; 35:6783–9. - PubMed
-
- Colombara DV, Khalil I, Rao PC, Troeger C, Forouzanfar MH, Riddle MS, Mokdad AH. Chronic health consequences of acute enteric infections in the developing world. Am J Gastroenterol Suppl 2016; 3:4–11.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

