Electrophysiological effects of nicotinic and electrical stimulation of intrinsic cardiac ganglia in the absence of extrinsic autonomic nerves in the rabbit heart
- PMID: 29800749
- PMCID: PMC6207532
- DOI: 10.1016/j.hrthm.2018.05.018
Electrophysiological effects of nicotinic and electrical stimulation of intrinsic cardiac ganglia in the absence of extrinsic autonomic nerves in the rabbit heart
Abstract
Background: The intrinsic cardiac nervous system is a rich network of cardiac nerves that converge to form distinct ganglia and extend across the heart and is capable of influencing cardiac function.
Objective: The goals of this study were to provide a complete picture of the neurotransmitter/neuromodulator profile of the rabbit intrinsic cardiac nervous system and to determine the influence of spatially divergent ganglia on cardiac electrophysiology.
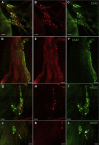
Methods: Nicotinic or electrical stimulation was applied at discrete sites of the intrinsic cardiac nerve plexus in the Langendorff-perfused rabbit heart. Functional effects on sinus rate and atrioventricular conduction were measured. Immunohistochemistry for choline acetyltransferase (ChAT), tyrosine hydroxylase, and/or neuronal nitric oxide synthase (nNOS) was performed using whole mount preparations.
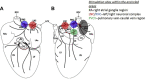
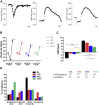
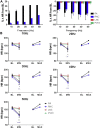
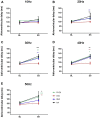
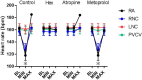
Results: Stimulation within all ganglia produced either bradycardia, tachycardia, or a biphasic brady-tachycardia. Electrical stimulation of the right atrial and right neuronal cluster regions produced the largest chronotropic responses. Significant prolongation of atrioventricular conduction was predominant at the pulmonary vein-caudal vein region. Neurons immunoreactive (IR) only for ChAT, tyrosine hydroxylase, or nNOS were consistently located within the limits of the hilum and at the roots of the right cranial and right pulmonary veins. ChAT-IR neurons were most abundant (1946 ± 668 neurons). Neurons IR only for nNOS were distributed within ganglia.
Conclusion: Stimulation of intrinsic ganglia, shown to be of phenotypic complexity but predominantly of cholinergic nature, indicates that clusters of neurons are capable of independent selective effects on cardiac electrophysiology, therefore providing a potential therapeutic target for the prevention and treatment of cardiac disease.
Keywords: Choline acetyltransferase; Electrophysiology; Ganglionic plexus; Heart; Intrinsic cardiac ganglia; Neuronal nitric oxide synthase; Tyrosine hydroxylase.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Wake E., Brack K. Characterization of the intrinsic cardiac nervous system. Auton Neurosci. 2016;199:3–16. - PubMed
-
- Butler C.K., Smith F.M., Cardinal R., Murphy D.A., Hopkins D.A., Armour J.A. Cardiac responses to electrical stimulation of discrete loci in canine atrial and ventricular ganglionated plexi. Am J Physiol. 1990;259:H1365–H1373. - PubMed
-
- Cardinal R., Page P., Vermeulen M., Ardell J.L., Armour J.A. Spatially divergent cardiac responses to nicotinic stimulation of ganglionated plexus neurons in the canine heart. Auton Neurosci. 2009;145:55–62. - PubMed
-
- Armour J.A., Huang M.H., Smith F.M. Peptidergic modulation of in situ canine intrinsic cardiac neurons. Peptides. 1993;14:191–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

